Injectable Drug Delivery Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430826 | Date : Nov, 2025 | Pages : 241 | Region : Global | Publisher : MRU
Injectable Drug Delivery Market Size
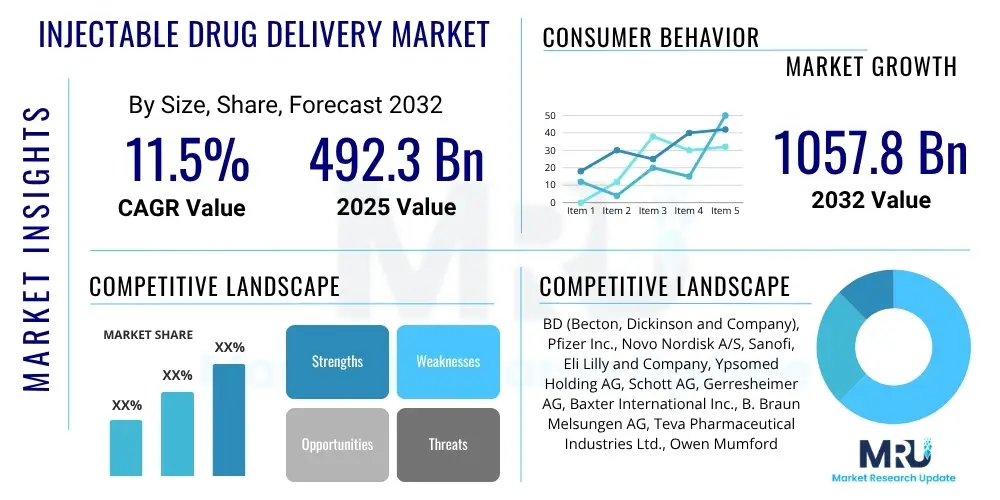
The Injectable Drug Delivery Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 11.5% between 2025 and 2032. The market is estimated at $492.3 Billion in 2025 and is projected to reach $1057.8 Billion by the end of the forecast period in 2032.
Injectable Drug Delivery Market introduction
The Injectable Drug Delivery Market encompasses a sophisticated array of medical devices and systems meticulously engineered for the precise and controlled administration of therapeutic agents directly into the body through injection. This critical sector of healthcare technology spans from conventional hypodermic syringes and vials to highly advanced, patient-friendly solutions such as prefilled syringes, auto-injectors, pen injectors, and sophisticated drug delivery pumps, including both ambulatory and implantable variants. The core objective of these diverse product offerings is to circumvent the limitations of oral drug administration, ensuring optimal bioavailability, rapid onset of action, and targeted delivery of medications. This is particularly vital for a growing class of complex biological drugs, vaccines, and potent small molecules that are either degraded by the digestive system or require immediate systemic access for therapeutic effect, thereby underpinning their indispensable role in modern medicine.
The major applications for injectable drug delivery systems are incredibly broad, addressing a vast spectrum of therapeutic areas critical to global public health. These applications include the long-term management of chronic conditions such as Type 1 and Type 2 diabetes, where insulin pen injectors and pumps are life-sustaining. They are fundamental in the treatment of various autoimmune disorders like rheumatoid arthritis and multiple sclerosis, requiring biologic therapies administered subcutaneously or intravenously. Furthermore, injectables are indispensable in oncology for delivering chemotherapy and targeted cancer drugs, in pain management for acute and chronic conditions, and globally for mass vaccination campaigns against infectious diseases. The benefits of these systems are manifold, significantly improving patient outcomes through enhanced drug efficacy, providing convenience and empowering patients through self-administration capabilities, and bolstering safety profiles by minimizing dosage errors and incorporating features to prevent needle-stick injuries. This comprehensive utility makes injectable drug delivery a cornerstone of contemporary medical practice.
The market's robust growth is propelled by a powerful combination of driving factors that reflect evolving healthcare needs and technological advancements. A primary driver is the accelerating global prevalence of chronic and lifestyle-related diseases, which often necessitate long-term, complex drug regimens best delivered via injection. Concurrently, the burgeoning biopharmaceutical industry, with its focus on biologics and biosimilars, inherently relies on injectable routes due to the molecular complexity and fragility of these drugs. The growing societal shift towards home healthcare and patient-centric treatment models further amplifies the demand for user-friendly self-administration devices, enhancing patient adherence and reducing the burden on clinical settings. Moreover, continuous technological innovations, encompassing smart features, improved ergonomics, and enhanced safety mechanisms, consistently expand the capabilities and appeal of injectable delivery systems, ensuring their sustained relevance and expansion in the global pharmaceutical landscape.
Injectable Drug Delivery Market Executive Summary
The Injectable Drug Delivery Market is navigating a period of dynamic evolution, characterized by significant shifts across business strategies, regional growth patterns, and segment-specific advancements. Business trends reveal an intensified focus on strategic alliances, mergers, and acquisitions among pharmaceutical companies and medical device manufacturers. These collaborations aim to integrate drug formulations with sophisticated delivery systems, accelerate product development, and expand market reach. A prominent shift is observed towards developing smart, connected injectable devices that offer real-time data tracking, adherence monitoring, and personalized dosage adjustments, aligning with the broader digital health transformation. Furthermore, there is an increasing emphasis on sustainable manufacturing practices and the development of eco-friendly device materials, driven by both regulatory pressures and corporate social responsibility initiatives, reshaping the competitive landscape and fostering innovation in product design and lifecycle management.
From a regional perspective, North America continues to command the largest share of the Injectable Drug Delivery Market, primarily due to its advanced healthcare infrastructure, substantial research and development investments, and high adoption rates of cutting-edge medical technologies. Europe also maintains a significant market presence, driven by an aging population, increasing chronic disease burden, and a strong emphasis on patient convenience and safety. However, the Asia Pacific region is rapidly emerging as the fastest-growing market, propelled by its enormous population base, improving economic conditions, expanding access to healthcare, and the increasing prevalence of lifestyle-related diseases. Countries such as China, India, and Japan are experiencing substantial growth, attracting foreign investments, and developing robust domestic manufacturing capabilities, positioning them as critical future growth engines for the global market. Latin America, the Middle East, and Africa are also showing promising growth, albeit from a lower base, as healthcare spending rises and awareness of advanced therapies increases.
Segment-wise, the market is witnessing robust expansion across several key areas. The prefilled syringes and auto-injectors segments are experiencing accelerated growth, largely attributed to their inherent benefits in terms of ease of use, dosage accuracy, and reduced risk of contamination, making them ideal for the self-administration of high-value biologics and biosimilars for chronic conditions like diabetes and autoimmune diseases. There is a growing demand for advanced drug delivery pumps, particularly for continuous infusion therapies that provide consistent drug levels. Moreover, the shift towards personalized medicine and precision dosing is driving innovation in formulation technologies, leading to more stable, concentrated, and patient-friendly injectable solutions. This evolving demand across segments underscores a market that is highly responsive to patient needs, technological breakthroughs, and the shifting landscape of pharmaceutical development, indicating sustained innovation and expansion into the foreseeable future.
AI Impact Analysis on Injectable Drug Delivery Market
Analysis of common user questions regarding the impact of Artificial Intelligence (AI) on the Injectable Drug Delivery Market reveals a broad spectrum of curiosity, tempered with cautious optimism. Users are keenly interested in how AI can fundamentally transform the drug development lifecycle, from accelerating the discovery of novel injectable drug candidates and optimizing their formulations for enhanced stability and delivery, to revolutionizing manufacturing processes for increased efficiency and reduced costs. There is significant inquiry into AI's role in personalizing treatment regimens, predicting patient responses to specific injectable therapies, and integrating smart functionalities into devices for improved adherence and safety. Concerns frequently emerge regarding data security and privacy within AI-driven systems, the regulatory pathways for AI-enabled medical devices, and the ethical considerations surrounding autonomous decision-making in patient care. Nonetheless, expectations are high for AI to usher in an era of unprecedented precision, predictive capability, and patient-centricity in injectable drug delivery, leading to more effective and safer therapeutic outcomes.
Artificial Intelligence is poised to exert a profound and transformative influence across the entire value chain of the Injectable Drug Delivery Market, fundamentally redefining how drugs are developed, delivered, and managed. In the realm of research and development, AI algorithms can analyze vast chemical and biological datasets to identify optimal drug candidates for injectable routes, predict their pharmacokinetic and pharmacodynamic profiles, and streamline the formulation process by optimizing excipients and delivery volumes. This accelerated discovery and development cycle reduces time to market and decreases associated costs. Furthermore, AI can aid in the design of novel delivery systems, such as advanced microneedles or implantable devices, by simulating drug release kinetics and device-tissue interactions, ensuring both efficacy and patient comfort. This predictive capability minimizes experimental iterations and accelerates innovation.
Beyond R&D, AI’s impact extends significantly to manufacturing, patient care, and post-market surveillance. In manufacturing, AI-powered systems can optimize production lines for injectable devices and prefilled syringes, enhancing quality control through anomaly detection, predicting equipment failures, and improving overall operational efficiency. For patient care, the integration of AI into smart injectable devices allows for real-time monitoring of physiological parameters, precise dosage adjustments based on individual patient needs, and personalized adherence tracking through connected platforms. This data-driven approach facilitates proactive intervention by healthcare providers, significantly improving therapeutic outcomes and reducing the incidence of adverse drug reactions. Additionally, AI can analyze large-scale real-world data from patient usage to identify emerging safety signals and provide valuable insights for product improvements, thereby continuously enhancing the safety and effectiveness of injectable drug delivery solutions throughout their lifecycle. This holistic integration of AI promises a future where injectable therapies are not only more effective but also intelligently adapted to individual patient journeys.
- AI-driven acceleration of drug discovery and development, identifying optimal candidates for injectable delivery.
- Optimization of drug formulations and excipient selection using AI models for enhanced stability and bioavailability.
- Integration of AI into smart injectable devices for real-time dosage monitoring, adherence tracking, and personalized treatment adjustments.
- Predictive analytics for patient response, allowing for customized therapy pathways and improved outcomes.
- Enhancement of manufacturing processes through AI-powered automation, quality control, and predictive maintenance.
- Development of intelligent algorithms for analyzing patient data, providing insights for disease management and prevention.
- AI-assisted design and simulation of novel injectable delivery systems, including advanced microneedle patches and implantables.
- Improved pharmacovigilance and post-market surveillance by leveraging AI to detect adverse events and safety signals from large datasets.
- Streamlining regulatory submission processes through AI-driven data compilation and analysis.
DRO & Impact Forces Of Injectable Drug Delivery Market
The Injectable Drug Delivery Market operates under the influence of a potent combination of drivers, restraints, and opportunities, which together define its impact forces and shape its growth trajectory. Key drivers include the escalating global prevalence of chronic diseases such as diabetes, autoimmune disorders, and various forms of cancer, all of which increasingly necessitate long-term, often self-administered, injectable drug therapies. The robust expansion of the biopharmaceutical industry, particularly the development and commercialization of complex biologics and biosimilars, inherently relies on injectable routes of administration due to the molecular fragility and specific pharmacokinetic requirements of these advanced medications. Furthermore, a significant societal shift towards patient-centric care models and the growing demand for convenient home healthcare options are powerfully driving the adoption of user-friendly self-administration devices, improving patient adherence and overall quality of life by empowering individuals to manage their conditions outside traditional clinical settings.
Conversely, the market faces several notable restraints that temper its growth. The high cost associated with the research, development, and sophisticated manufacturing of advanced injectable drug delivery systems can limit widespread accessibility, particularly in price-sensitive emerging markets. Concerns surrounding the risk of needle-stick injuries for both healthcare professionals and patients continue to necessitate the development of safety-engineered devices, adding to manufacturing complexities and costs. Stringent and evolving regulatory approval processes for novel drug-device combination products often extend timelines and increase the financial burden on manufacturers, creating barriers to market entry for new innovations. Additionally, the need for adequate training for patients on self-injection techniques and for healthcare providers on the proper use of complex devices can pose logistical challenges, particularly in regions with limited healthcare infrastructure or skilled personnel, hindering optimal market penetration.
Despite these challenges, the Injectable Drug Delivery Market is rich with significant opportunities that promise to drive future growth and innovation. The burgeoning interest in personalized medicine presents a substantial opportunity for the development of highly customized injectable therapies and smart delivery devices capable of tailoring dosages to individual patient needs based on real-time physiological data. The expansion into emerging economies, characterized by improving healthcare infrastructures, rising disposable incomes, and increasing awareness of advanced medical treatments, offers vast untapped market potential. Furthermore, continuous research and development into novel drug formulations, such as sustained-release injectables that reduce dosing frequency, and advanced delivery technologies like microneedle patches and innovative implantable pumps, are poised to revolutionize patient experience and therapeutic outcomes. These opportunities, driven by scientific progress and evolving patient demands, represent powerful forces that will continue to shape and expand the market, compelling stakeholders to innovate and adapt to meet future healthcare challenges effectively.
Segmentation Analysis
The Injectable Drug Delivery Market is comprehensively segmented to provide a granular understanding of its diverse components, facilitating a detailed analysis of market dynamics, competitive landscapes, and future growth trajectories. This intricate segmentation allows for the precise identification of various product types, ranging from basic syringes to highly advanced automated devices, each designed for specific administration needs. Further distinctions are made based on the therapeutic areas these systems target, reflecting the critical role injectables play across a multitude of medical conditions. The market is also analyzed from the perspective of end-users, differentiating between institutional settings and home-based care, which significantly impacts device design and distribution strategies. Lastly, the segmentation by route of administration is crucial, as it dictates the technical specifications and clinical applications of each injectable solution, offering a multi-dimensional view of this vital healthcare sector.
This detailed market segmentation is essential for stakeholders to discern specific trends and allocate resources effectively. For example, the product segment highlights the rapid shift towards prefilled syringes and auto-injectors due to their convenience and safety features, especially relevant for the burgeoning biologics market. Understanding therapeutic area segments allows manufacturers to tailor devices for conditions like diabetes or autoimmune disorders, addressing unique patient needs and regulatory requirements. The distinction between end-user environments underscores the growing importance of user-friendly devices for self-administration in home care settings, driven by an aging population and a preference for independent disease management. Each segment offers distinct growth opportunities and challenges, and a thorough analysis of their interdependencies provides a holistic insight into the market’s current state and its potential future developments, enabling strategic decision-making for innovation and market expansion.
- By Product Type
- Devices
- Syringes
- Conventional Syringes
- Safety Syringes (with retractable needles, needle shields)
- Auto-injectors (for emergency use, chronic conditions)
- Pen Injectors (for diabetes, growth hormones)
- Prefilled Syringes (enhancing patient safety and convenience)
- Drug Delivery Pumps
- Ambulatory Pumps (for continuous infusion, pain management)
- Implantable Pumps (for long-term drug delivery, pain management, cancer)
- Patch Pumps (wearable, for insulin and other therapies)
- Needle-Free Injectors (minimizing phobia, reducing needle-stick injuries)
- Microneedle Patches (for transdermal delivery, vaccines, biologics)
- Syringes
- Formulations
- Liquid Formulations (ready-to-use solutions)
- Powder Reconstitution (lyophilized drugs, requiring dilution)
- Suspensions (insoluble drug particles dispersed in liquid)
- Emulsions (oil-in-water or water-in-oil systems)
- Liposomes (for targeted drug delivery, reduced toxicity)
- Nanoparticle Formulations (enhanced drug solubility, bioavailability)
- Devices
- By Therapeutic Area
- Autoimmune Diseases (e.g., Rheumatoid Arthritis, Multiple Sclerosis, Psoriasis, Crohn's Disease)
- Cancer (e.g., Chemotherapy, Targeted Biologics)
- Diabetes (e.g., Insulin, GLP-1 agonists)
- Pain Management (e.g., Opioids, NSAIDs, local anesthetics)
- Hormonal Disorders (e.g., Growth Hormone Deficiency, Infertility)
- Infectious Diseases (e.g., Antibiotics, Antivirals, Vaccines)
- Cardiovascular Diseases (e.g., Anticoagulants, Vasodilators)
- Neurological Disorders (e.g., Parkinson's Disease, Epilepsy)
- Vaccines (e.g., Influenza, Hepatitis, COVID-19)
- Ophthalmology (e.g., Anti-VEGF for macular degeneration)
- By End User
- Hospitals and Clinics (large volume usage, diverse applications)
- Home Care Settings (growing segment, driven by self-administration)
- Long-term Care Facilities (elderly care, chronic illness management)
- Ambulatory Surgical Centers (outpatient procedures, rapid recovery)
- Physician Offices (routine injections, vaccinations)
- Pharmaceutical & Biotechnology Companies (for clinical trials, drug development)
- By Route of Administration
- Subcutaneous (SC) (convenient for self-administration, biologics)
- Intramuscular (IM) (vaccines, certain medications)
- Intravenous (IV) (rapid onset, high bioavailability, emergency care)
- Intradermal (ID) (allergy testing, certain vaccines)
- Intra-articular (directly into joints for pain or inflammation)
- Intraperitoneal (into the abdominal cavity, specific cancer treatments)
- Intrathecal (into the spinal canal, pain management, neurological conditions)
Value Chain Analysis For Injectable Drug Delivery Market
The value chain for the Injectable Drug Delivery Market represents a sophisticated and highly regulated ecosystem, starting from the meticulous sourcing of raw materials and extending to the final delivery of sophisticated devices to end-users. The upstream segment of the value chain is critical and involves the procurement of highly specialized components. This includes pharmaceutical-grade glass or plastic for vials and prefilled syringe barrels, high-quality stainless steel for needles, advanced polymers for plungers and device housings, and sophisticated electronic components for smart, connected injectors. Key participants in this stage are specialized material suppliers and precision component manufacturers who must adhere to rigorous quality control standards, biocompatibility requirements, and international regulatory guidelines, as any deviation can compromise product safety and efficacy, which forms the bedrock of patient trust and product performance.
Moving further along the chain, the midstream activities encompass the intricate processes of design, development, and manufacturing of the injectable drug delivery devices themselves. This phase is dominated by medical device companies, specialized contract manufacturing organizations (CMOs), and increasingly, integrated pharmaceutical companies that have in-house device development capabilities. It involves sophisticated engineering for device assembly, integration of drug formulations into sterile prefilled systems, and extensive validation and verification testing to ensure reliability, accuracy, and safety. Innovation at this stage focuses on ergonomic design, enhanced safety features like needle-stick prevention, and the incorporation of advanced technologies such as connectivity and data analytics, all while navigating complex intellectual property landscapes and regulatory approvals from bodies like the FDA and EMA.
The downstream segment of the value chain is focused on efficient distribution and market access for these finished injectable products. Distribution channels can be broadly categorized into direct and indirect models. Direct distribution involves manufacturers selling directly to major hospitals, integrated healthcare systems, large pharmacy chains, or strategic pharmaceutical partners, allowing for tighter control over inventory and customer relationships. Indirect distribution, conversely, leverages a vast network of wholesalers, third-party logistics (3PL) providers, specialized medical distributors, and group purchasing organizations (GPOs) to reach a broader and more geographically dispersed customer base, including smaller clinics, retail pharmacies, and home care agencies. Both channels require robust supply chain management, cold chain capabilities for temperature-sensitive biologics, and compliance with intricate import/export regulations to ensure that critical injectable therapies are delivered safely, efficiently, and promptly to patients worldwide.
Injectable Drug Delivery Market Potential Customers
The Injectable Drug Delivery Market caters to a diverse and expanding base of potential customers, each with unique requirements and purchasing motivations that influence product development and market strategies. At the forefront are individual patients, particularly those managing chronic conditions such as diabetes, autoimmune disorders, various cancers, or hormonal imbalances, who require frequent or long-term injectable medications. These end-users prioritize ease of use, minimal pain during injection, accurate dosing, portability, and discreet design, all of which contribute significantly to improved adherence and enhanced quality of life by enabling self-administration in the comfort of their homes. The growing global aging population further expands this demographic, as older adults often manage multiple chronic conditions that necessitate injectable therapies, seeking devices that are intuitive and ergonomically designed for their needs.
Another critical segment of potential customers comprises healthcare providers, including a wide array of institutions such as hospitals, clinics, ambulatory surgical centers, and long-term care facilities. These professional buyers procure injectable drug delivery systems for a vast range of applications, from routine vaccinations and emergency medical interventions to complex surgical procedures and inpatient therapeutic regimens. Their purchasing decisions are primarily driven by factors such as device safety (e.g., features to prevent needle-stick injuries), cost-effectiveness over time, compatibility with various drug formulations, sterility assurance, and seamless integration into existing clinical workflows and medical equipment. Efficiency, reliability, and stringent adherence to healthcare regulations are paramount considerations for these institutional customers, impacting their selection of suppliers and specific device technologies to ensure optimal patient care and operational effectiveness.
Furthermore, pharmaceutical and biotechnology companies constitute a substantial and strategic customer segment, as they increasingly seek innovative and reliable drug delivery solutions for their burgeoning pipelines, especially for biologics and biosimilars. These companies partner with device manufacturers to develop integrated drug-device combination products that optimize drug stability, improve bioavailability, and enhance patient acceptance, thereby gaining a competitive edge in a highly regulated market. Their focus lies on scalable manufacturing, regulatory compliance, intellectual property protection, and ensuring that the chosen delivery system complements the unique characteristics of their drug product, ultimately aiming to maximize therapeutic efficacy and market penetration. Additionally, government health organizations, non-governmental organizations (NGOs), and public health initiatives represent significant buyers, particularly for large-scale vaccination programs and disease eradication efforts, where product affordability, widespread availability, and robust supply chain logistics are key determinants in procurement decisions.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $492.3 Billion |
| Market Forecast in 2032 | $1057.8 Billion |
| Growth Rate | 11.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | BD (Becton, Dickinson and Company), Pfizer Inc., Novo Nordisk A/S, Sanofi, Eli Lilly and Company, Ypsomed Holding AG, Schott AG, Gerresheimer AG, Baxter International Inc., B. Braun Melsungen AG, Teva Pharmaceutical Industries Ltd., Owen Mumford Ltd., SHL Medical AG, West Pharmaceutical Services Inc., Terumo Corporation, Haselmeier GmbH, C. R. Bard Inc. (part of BD), Unilife Corporation, Alkermes Plc, Consort Medical plc (part of Recipharm AB), Enable Injections, Stevanato Group, Catalent Inc., Nemera, Eitan Medical, Medtronic plc, Valeritas, Inc. (now part of Zealand Pharma), Insulet Corporation, Antares Pharma, Inc. (now part of Halozyme Therapeutics), Conmed Corporation, Smiths Group plc, Vetter Pharma-Fertigung GmbH & Co. KG, Lonza Group Ltd., WuXi AppTec Co. Ltd., Sartorius AG |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Injectable Drug Delivery Market Key Technology Landscape
The technological landscape of the Injectable Drug Delivery Market is characterized by relentless innovation aimed at significantly enhancing patient experience, improving drug efficacy, and ensuring optimal safety and compliance. A cornerstone of this landscape involves the continuous refinement and expansion of prefilled syringe technology, which minimizes preparation steps, reduces the risk of contamination, and offers precise, pre-measured doses crucial for sensitive biologics. Complementing this are advancements in auto-injectors and pen injectors, which are engineered for user-friendliness, ergonomic design, and integration of safety features such as passive needle shielding, making self-administration simpler and safer for patients managing chronic conditions like diabetes or autoimmune disorders at home, thereby increasing adherence and reducing healthcare burdens.
Emerging technologies are increasingly focused on connectivity and intelligence, ushering in the era of smart injectable devices. These sophisticated systems integrate sensors, microprocessors, and wireless communication capabilities (e.g., Bluetooth) to monitor dosage delivery, track patient adherence patterns, and transmit vital data to companion smartphone applications or healthcare provider platforms. This digital integration enables personalized treatment adjustments, provides valuable real-time feedback to patients, and allows healthcare professionals to monitor therapeutic effectiveness remotely. Such advancements are pivotal for optimizing chronic disease management and supporting the broader trend towards digital health and remote patient monitoring, transforming how injectable therapies are managed and experienced by patients.
Further driving innovation are developments in alternative, less invasive delivery methods and advanced drug formulations designed for prolonged release. Microneedle patches offer a revolutionary approach to transdermal drug delivery, allowing for the pain-free administration of vaccines, insulin, and other biologics through tiny, superficial needles, appealing particularly to needle-phobic patients. Needle-free injection systems, using high-pressure streams to deliver medication, also address needle anxiety and reduce the risk of accidental needle-stick injuries. Concurrently, advancements in drug formulation, including sustained-release injectables utilizing biodegradable polymers, liposomes, or nanoparticles, aim to reduce the frequency of injections, thereby improving patient convenience and therapeutic consistency. Implantable drug delivery pumps represent another frontier, offering highly controlled, long-term drug administration for complex conditions like chronic pain or certain cancers. These diverse technological thrusts collectively underscore a dynamic market committed to evolving injectable therapies for enhanced patient outcomes and accessibility.
Regional Highlights
- North America: This region consistently maintains the largest share of the Injectable Drug Delivery Market, propelled by several robust factors. These include a highly advanced and well-funded healthcare infrastructure, significant investments in pharmaceutical and biotechnology research and development, and a high adoption rate of sophisticated medical technologies. The substantial prevalence of chronic diseases, coupled with favorable reimbursement policies and a strong emphasis on personalized medicine and patient convenience, further fuels market expansion. The United States, in particular, stands as a global leader in innovation for smart injectable devices and self-administration solutions, driving significant revenue and technological advancements across the region.
- Europe: Europe represents a mature yet continually growing market for injectable drug delivery, underpinned by an increasingly aging population, a high incidence of chronic diseases, and a steadfast commitment to patient safety and quality of care. Countries such as Germany, France, and the UK are at the forefront of adopting advanced injectable delivery systems, including prefilled syringes, auto-injectors, and connected devices, driven by proactive governmental healthcare initiatives and strong regulatory frameworks that encourage innovation. The region's robust pharmaceutical industry and extensive healthcare expenditure contribute significantly to sustained market expansion and the rapid integration of new delivery technologies.
- Asia Pacific (APAC): The Asia Pacific region is unequivocally projected to exhibit the fastest growth rate in the Injectable Drug Delivery Market over the forecast period. This accelerated growth is primarily attributed to a massive and expanding population base, significant improvements in healthcare infrastructure, rising disposable incomes, and a burgeoning awareness of advanced medical treatments. Key emerging economies such as China, India, and Japan are investing heavily in modernizing their healthcare systems, fostering local manufacturing capabilities, and witnessing a surge in demand for affordable, accessible, and high-quality injectable solutions. The rapid expansion of the biopharmaceutical sector within APAC further acts as a powerful catalyst for regional market development, making it a critical hub for future market opportunities.
- Latin America: This region is experiencing a steady and promising growth trajectory in the Injectable Drug Delivery Market. Factors contributing to this expansion include increasing healthcare expenditure across several nations, improving access to medical facilities, and a rising prevalence of chronic and infectious diseases requiring effective injectable therapies. While still a developing market compared to the more established regions, countries like Brazil, Mexico, and Argentina are leading the way, driven by governmental initiatives aimed at enhancing public health, expanding vaccination programs, and improving the availability of essential medicines. The growing middle class and evolving regulatory landscapes also play a pivotal role in fostering market development in Latin America.
- Middle East and Africa (MEA): The MEA region represents a nascent but rapidly evolving market for injectable drug delivery, characterized by considerable long-term potential. Growth is being propelled by substantial healthcare investments, particularly within the Gulf Cooperation Council (GCC) countries, focusing on modernizing healthcare infrastructure and diversifying economies away from oil dependence. The increasing burden of both non-communicable and infectious diseases necessitates improved drug delivery solutions. However, challenges such as economic instability, varying levels of healthcare access, and fragmented regulatory environments in some parts of Africa temper the overall growth rate, though significant opportunities exist for basic, essential, and increasingly advanced injectable solutions as healthcare systems mature and become more integrated.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Injectable Drug Delivery Market.- BD (Becton, Dickinson and Company)
- Pfizer Inc.
- Novo Nordisk A/S
- Sanofi
- Eli Lilly and Company
- Ypsomed Holding AG
- Schott AG
- Gerresheimer AG
- Baxter International Inc.
- B. Braun Melsungen AG
- Teva Pharmaceutical Industries Ltd.
- Owen Mumford Ltd.
- SHL Medical AG
- West Pharmaceutical Services Inc.
- Terumo Corporation
- Haselmeier GmbH
- C. R. Bard Inc. (part of BD)
- Unilife Corporation
- Alkermes Plc
- Consort Medical plc (part of Recipharm AB)
- Dover Corporation (through various subsidiaries)
- Amgen Inc.
- Roche Holding AG
- Merck & Co., Inc.
- AbbVie Inc.
- Medtronic plc
- Valeritas, Inc. (now part of Zealand Pharma)
- Insulet Corporation
- Antares Pharma, Inc. (now part of Halozyme Therapeutics)
- Enable Injections
- Stevanato Group
- Catalent Inc.
- Nemera
- Eitan Medical
- Conmed Corporation
- Smiths Group plc
- Vetter Pharma-Fertigung GmbH & Co. KG
- Lonza Group Ltd.
- WuXi AppTec Co. Ltd.
- Sartorius AG
Frequently Asked Questions
What are the primary drivers of growth in the Injectable Drug Delivery Market?
The primary drivers propelling the Injectable Drug Delivery Market include the escalating global prevalence of chronic diseases such as diabetes, autoimmune disorders, and various cancers, which necessitate frequent or long-term injectable therapies. Furthermore, the robust growth of the biopharmaceutical industry, with its focus on complex biologics and biosimilars, inherently relies on injectable routes for effective administration. The increasing demand for patient-friendly self-administration devices that enhance convenience and adherence, coupled with continuous technological advancements in device design and smart features, are also significant catalysts for market expansion.
How is technological innovation impacting injectable drug delivery systems?
Technological innovation is profoundly transforming the market by introducing sophisticated solutions that prioritize patient safety, convenience, and efficacy. This includes the widespread adoption of advanced auto-injectors and pen injectors designed for simpler self-administration, along with prefilled syringes that minimize medication errors and preparation time. Crucially, the integration of smart features into these devices, such as connectivity for real-time dosage monitoring and adherence tracking, is enabling personalized treatment regimens and facilitating remote patient management, thereby significantly improving therapeutic outcomes and patient engagement.
What are the key challenges faced by the Injectable Drug Delivery Market?
The Injectable Drug Delivery Market encounters several key challenges, notably the high costs associated with research, development, and sophisticated manufacturing of advanced delivery systems, which can limit widespread accessibility. Stringent regulatory approval processes for novel drug-device combination products often extend timelines and increase financial burdens on manufacturers. Concerns regarding needle-stick injuries and the safe disposal of sharps also persist, driving the need for continuous innovation in safety-engineered devices. Additionally, ensuring adequate training for both patients in self-injection techniques and healthcare professionals in device handling remains a logistical hurdle in various regions.
Which therapeutic areas are the largest consumers of injectable drug delivery systems?
The largest therapeutic areas significantly relying on injectable drug delivery systems include diabetes, where insulin and GLP-1 agonists are predominantly administered via pen injectors or pumps; autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and psoriasis, often treated with biologic therapies; and oncology, for delivering chemotherapy and targeted cancer drugs. Infectious diseases, particularly through large-scale vaccination programs, and chronic pain management also represent substantial and growing application segments due to the need for precise and effective drug delivery.
What role does home care play in the growth of the Injectable Drug Delivery Market?
Home care plays a pivotal role in the expansion of the Injectable Drug Delivery Market by fueling the demand for user-friendly, self-administration devices. This trend empowers patients with chronic conditions to manage their therapies independently outside of traditional clinical settings, thereby enhancing convenience, privacy, and quality of life while simultaneously reducing the burden on healthcare facilities. The development of intuitive auto-injectors, pen injectors, and even patch pumps specifically designed for home use, coupled with digital health integration for remote monitoring, is a key driver for market growth and the shift towards decentralized healthcare models.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
