Intermittent Catheters Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430049 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Intermittent Catheters Market Size
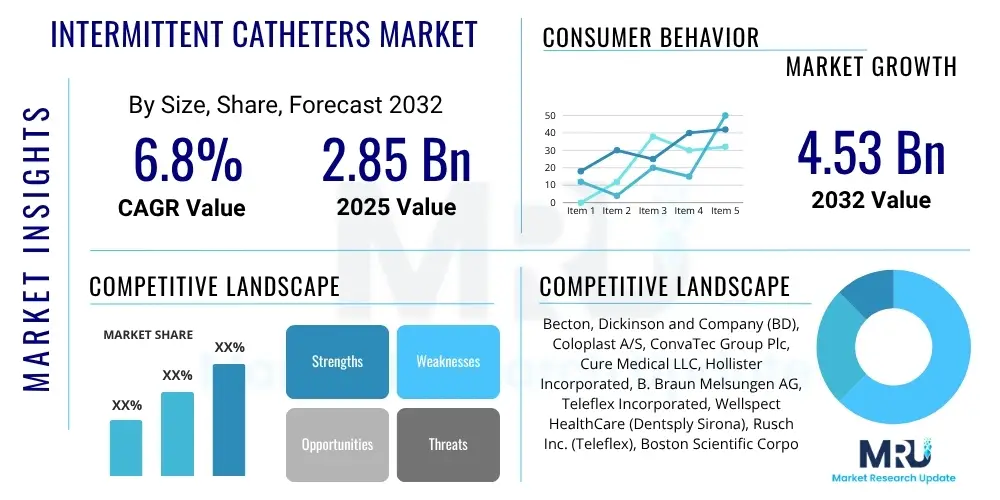
The Intermittent Catheters Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 2.85 Billion in 2025 and is projected to reach USD 4.53 Billion by the end of the forecast period in 2032.
Intermittent Catheters Market introduction
The Intermittent Catheters Market serves a critical role in managing urinary incontinence and retention for a vast patient population globally. These medical devices are specifically designed for temporary bladder drainage, allowing individuals to manage their condition independently and improve their quality of life. Product descriptions encompass a range of designs, from advanced hydrophilic-coated catheters that significantly reduce friction and insertion discomfort, to those made from biocompatible materials like silicone and PVC, available in various sizes, lengths, and tip configurations such as straight, coude, or Tiemann to suit diverse anatomical and clinical needs. Major applications span across patients with neurological conditions such as spinal cord injuries, multiple sclerosis, and spina bifida, as well as individuals experiencing post-operative urinary retention, non-neurogenic bladder dysfunction, or those requiring intermittent drainage for other urological issues. The inherent benefits of intermittent catheterization include a significant reduction in the risk of urinary tract infections (UTIs) compared to indwelling catheters, enhanced patient autonomy, greater mobility, and improved social participation due to discreet and convenient use. This market's robust growth is primarily driven by an aging global demographic, which inherently leads to an increased prevalence of age-related urological disorders, coupled with continuous technological advancements aimed at improving user comfort, safety, and ease of use, alongside growing awareness and acceptance of self-catheterization as a viable long-term solution for bladder management. Furthermore, the shift towards home-based care and outpatient settings increasingly favors the adoption of these user-friendly medical devices, supporting a more independent lifestyle for patients.
Intermittent Catheters Market Executive Summary
The Intermittent Catheters Market is experiencing dynamic growth, propelled by several key business, regional, and segment trends. Business trends are characterized by a strong focus on product innovation, with manufacturers investing heavily in developing ultra-smooth hydrophilic coatings, compact and discreet packaging, and improved ergonomic designs that enhance user comfort and reduce the risk of complications. There is also a notable trend towards strategic partnerships and collaborations among market players to expand distribution networks and leverage technological expertise, alongside increasing integration of patient education and support services. Regional trends indicate that North America and Europe continue to be dominant markets due due to advanced healthcare infrastructures, high awareness levels, and supportive reimbursement policies; however, the Asia Pacific region is rapidly emerging as a high-growth market, driven by improving healthcare access, a large and aging population, and rising incidence of chronic diseases contributing to urinary dysfunction. Latin America and the Middle East & Africa regions are also showing promising growth, albeit from a lower base, as healthcare spending and awareness increase. Segment trends highlight the growing preference for hydrophilic and pre-lubricated catheters over uncoated alternatives, primarily due to their superior comfort and reduced risk of urethral trauma and UTIs. The demand for gender-specific designs is also increasing, reflecting a more patient-centric approach to product development. Furthermore, the homecare settings segment is projected to grow significantly faster than hospital settings, underscoring the shift towards self-management and independent living facilitated by convenient intermittent catheter solutions.
AI Impact Analysis on Intermittent Catheters Market
Common user questions regarding AI's impact on the Intermittent Catheters Market often revolve around the potential for personalized patient care, enhanced product design, improved manufacturing processes, and predictive analytics for complications. Users are keen to understand if AI can make catheterization safer, more efficient, and more tailored to individual needs. This analysis suggests that AI holds significant promise for transforming the intermittent catheters landscape by enabling more intelligent solutions and better patient outcomes. Specifically, AI's influence is expected to manifest in several key areas, from optimizing material science for next-generation catheter designs to providing smart, personalized guidance for patients, ultimately making the entire process of intermittent catheterization more intuitive and effective while mitigating common risks.
- AI can facilitate personalized catheter selection by analyzing patient-specific anatomical data, lifestyle, and medical history, recommending the most suitable catheter type, size, and material, thereby improving comfort and efficacy.
- Predictive analytics powered by AI can help identify patients at higher risk of developing urinary tract infections or other complications, allowing for proactive intervention and personalized care plans, potentially reducing healthcare burdens.
- AI can optimize manufacturing processes through machine learning algorithms for quality control, defect detection, and supply chain management, leading to more efficient production, reduced waste, and improved product consistency.
- Integration of AI with smart devices and telehealth platforms can provide patients with real-time feedback, personalized usage reminders, and virtual training, enhancing adherence to catheterization schedules and proper technique.
- Development of smart, sensor-integrated catheters could utilize AI to monitor bladder volume, fluid flow, and even detect early signs of infection, providing data-driven insights for both patients and healthcare providers.
- AI could aid in the design of next-generation catheters by simulating fluid dynamics and material stress, leading to innovations in coating technology and ergonomic forms that maximize patient comfort and safety.
DRO & Impact Forces Of Intermittent Catheters Market
The Intermittent Catheters Market is significantly shaped by a complex interplay of drivers, restraints, opportunities, and broader impact forces. Key drivers include the escalating global prevalence of chronic urological disorders such as neurogenic bladder and urinary retention, largely attributable to an aging population and a rising incidence of conditions like spinal cord injuries, multiple sclerosis, and prostate cancer. Increasing awareness and acceptance of intermittent self-catheterization (ISC) as a safe and effective long-term bladder management solution, particularly given its benefits in reducing complications like UTIs compared to indwelling catheters, further propels market expansion. Moreover, continuous technological advancements in catheter design, such as the development of ultra-smooth hydrophilic coatings, compact and discreet packaging, and pre-lubricated options, significantly enhance user comfort, ease of use, and adherence, thereby stimulating demand. However, the market faces notable restraints, including the persistent risk of complications like urethral trauma, strictures, and urinary tract infections, which can deter some patients and healthcare providers. The high cost associated with advanced, specialized intermittent catheters, especially in price-sensitive developing regions, also limits widespread adoption. Additionally, a lack of adequate awareness and training in some geographical areas, coupled with existing regulatory hurdles and varying reimbursement policies, can impede market growth. Opportunities abound in the development of antimicrobial catheter coatings to minimize infection risks, the expansion into emerging markets with rapidly improving healthcare infrastructures and growing patient pools, and the integration of digital health solutions to provide better patient education and remote support. The market is also influenced by impact forces such as the bargaining power of buyers, driven by increased product availability and focus on cost-effectiveness, and the bargaining power of suppliers, influenced by the specialized nature of raw materials. The threat of new entrants remains moderate due to regulatory requirements and R&D investment, while the threat of substitutes is low given the medical necessity and established efficacy of intermittent catheters. Intense competitive rivalry among key players drives innovation and market penetration strategies, ensuring continuous product improvement and market dynamism.
Segmentation Analysis
The Intermittent Catheters Market is comprehensively segmented based on various critical parameters, including product type, material, end user, and gender, providing a granular view of market dynamics and consumer preferences. These segmentations are crucial for understanding demand patterns, identifying growth opportunities, and tailoring product development and marketing strategies to specific patient populations and healthcare settings. Each segment exhibits distinct characteristics and growth trajectories, reflecting diverse clinical needs and technological advancements across the global healthcare landscape.
- By Product Type:
- Coated Intermittent Catheters
- Hydrophilic Coated Catheters
- Anti-bacterial Coated Catheters
- Other Coated Catheters
- Uncoated Intermittent Catheters
- Coated Intermittent Catheters
- By Material:
- PVC (Polyvinyl Chloride)
- Silicone
- Latex
- Other Materials (e.g., Polyurethane)
- By End User:
- Hospitals
- Homecare Settings
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics
- By Gender:
- Male Catheters
- Female Catheters
- Pediatric Catheters
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America
- Middle East and Africa (MEA)
Value Chain Analysis For Intermittent Catheters Market
The value chain for the Intermittent Catheters Market is a multi-stage process encompassing raw material sourcing, manufacturing, distribution, and end-user engagement, with each stage contributing significantly to the final product's value and accessibility. The upstream analysis involves the procurement of specialized raw materials such as medical-grade PVC, silicone, polyurethane, and advanced hydrophilic coating compounds from various chemical and polymer suppliers. These suppliers must adhere to stringent quality and regulatory standards to ensure product safety and biocompatibility. Following material sourcing, manufacturers design and produce the catheters, involving complex molding, extrusion, coating, and assembly processes, often within highly controlled cleanroom environments, alongside rigorous sterilization and packaging procedures. Downstream analysis focuses on the channels through which these catheters reach the end-users. Distribution channels are varied, including direct sales from manufacturers to large hospitals and government healthcare systems, and indirect sales facilitated by a network of wholesalers, distributors, and group purchasing organizations (GPOs) that manage logistics and supply to smaller clinics, pharmacies, and homecare providers. The increasing prominence of e-commerce platforms and online pharmacies also represents a growing direct-to-consumer distribution channel, particularly for homecare users seeking convenience and discretion. The intricate coordination across these stages, from the selection of superior raw materials to efficient delivery through direct and indirect channels, is crucial for market penetration, product availability, and ultimately, patient satisfaction and safety in the intermittent catheters market.
Intermittent Catheters Market Potential Customers
The potential customers for the Intermittent Catheters Market primarily consist of individuals who experience various forms of urinary retention or incontinence, necessitating regular bladder drainage, along with the healthcare professionals and caregivers who support them. This broad end-user base includes a significant demographic of patients with chronic neurological conditions such as spinal cord injury, multiple sclerosis, spina bifida, and Parkinson's disease, all of whom often develop neurogenic bladder dysfunction requiring intermittent catheterization for effective bladder management. Additionally, a substantial portion of the customer base comprises individuals undergoing post-operative recovery, particularly following urological or pelvic surgeries, where temporary urinary retention is common. Patients with benign prostatic hyperplasia (BPH) causing urinary outflow obstruction, or those with non-neurogenic urinary retention due to medication side effects or functional issues, also represent key end-users. Beyond individual patients, the buyers of these products include a wide array of healthcare institutions such as hospitals, rehabilitation centers, long-term care facilities, and ambulatory surgical centers that stock and dispense catheters. Furthermore, homecare providers, pharmacies, and increasingly, direct-to-consumer online medical supply retailers serve as crucial touchpoints for patients and their caregivers who manage self-catheterization in their daily lives. The market also caters to pediatric patients with congenital conditions affecting bladder function, highlighting the need for age and size-specific catheter solutions. Understanding the diverse needs and procurement channels of these varied potential customers is vital for market players to effectively develop and distribute intermittent catheter products.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 2.85 Billion |
| Market Forecast in 2032 | USD 4.53 Billion |
| Growth Rate | CAGR 6.8% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Becton, Dickinson and Company (BD), Coloplast A/S, ConvaTec Group Plc, Cure Medical LLC, Hollister Incorporated, B. Braun Melsungen AG, Teleflex Incorporated, Wellspect HealthCare (Dentsply Sirona), Rusch Inc. (Teleflex), Boston Scientific Corporation, Medline Industries, LP, Rochester Medical Corporation (C.R. Bard), Manfred Sauer GmbH, CompactCath, LoFric (Wellspect HealthCare), Pennine Healthcare, Urocare Products Inc., Hunter Urology, PFM Medical, Mentis Corporation |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Intermittent Catheters Market Key Technology Landscape
The Intermittent Catheters Market is continually evolving, driven by significant advancements in material science, design engineering, and user-centric features, all aimed at enhancing patient comfort, reducing complications, and promoting ease of use. A cornerstone of modern intermittent catheter technology is the development and widespread adoption of hydrophilic coating technology. These coatings, activated by water, create an ultra-slippery surface that significantly minimizes friction during insertion and withdrawal, thereby reducing the risk of urethral trauma, discomfort, and long-term complications such as strictures. Another critical technological advancement involves the use of advanced, biocompatible low-friction materials like medical-grade silicone and polyurethane, which offer superior flexibility and softness compared to traditional PVC, further improving patient safety and comfort while also addressing concerns about phthalates. Manufacturers are also increasingly focusing on anti-bacterial coating technologies, often incorporating silver ions or other antimicrobial agents, to actively inhibit bacterial growth on the catheter surface and substantially reduce the incidence of catheter-associated urinary tract infections (CAUTIs), which remain a major concern for long-term users. Furthermore, product innovation extends to compact and discreet designs, such as pocket-sized catheters and integrated water sachets for immediate activation of hydrophilic coatings, which provide unparalleled convenience and discretion for users, promoting greater independence and adherence to self-catheterization routines. The emergence of 'no-touch' handling systems, where the catheter can be inserted without direct hand contact, significantly enhances hygiene and further minimizes infection risks. Looking ahead, there is a growing trend towards smart catheter concepts, potentially integrating sensors for real-time bladder monitoring or developing biodegradable materials, signaling a future of even more sophisticated and patient-friendly solutions in this critical medical device sector.
Regional Highlights
- North America: This region stands as a dominant force in the Intermittent Catheters Market, characterized by its well-established healthcare infrastructure, high awareness regarding bladder management solutions, and favorable reimbursement policies. The presence of a large aging population, coupled with a high prevalence of chronic neurological disorders and spinal cord injuries, significantly drives demand. Extensive research and development activities, along with the early adoption of advanced medical technologies and patient-centric care models, contribute to the region's market leadership. The United States, in particular, leads in terms of market size and innovation, with Canada also demonstrating robust growth.
- Europe: Europe represents another significant market for intermittent catheters, largely driven by an increasingly aging demographic and a high incidence of conditions such as multiple sclerosis and spina bifida, which necessitate long-term bladder management. Strong emphasis on home-based care and patient independence, coupled with supportive healthcare systems and established clinical guidelines for intermittent self-catheterization, underpins market growth. Countries like Germany, the UK, France, and the Nordic nations are key contributors, known for high product adoption and continuous innovation in catheter design and materials.
- Asia Pacific (APAC): The Asia Pacific region is rapidly emerging as the fastest-growing market for intermittent catheters. This growth is fueled by improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness of advanced medical treatments. A large and expanding population base, particularly an aging demographic in countries like Japan and China, coupled with a growing prevalence of lifestyle-related diseases and neurological conditions, contributes substantially to market expansion. Economic development and increasing disposable incomes are making advanced catheter solutions more accessible, while localized manufacturing and distribution efforts are boosting market penetration across the region.
- Latin America: The Latin American market for intermittent catheters is experiencing steady growth, primarily attributed to increasing access to healthcare services, improving diagnostic capabilities for urological conditions, and a rising awareness among both patients and healthcare professionals about effective bladder management options. Countries such as Brazil, Mexico, and Argentina are leading this growth, driven by efforts to modernize healthcare systems and address the growing burden of chronic diseases. However, market expansion faces challenges related to economic disparities and varying healthcare policies, requiring localized strategies for market penetration.
- Middle East and Africa (MEA): The MEA region is a nascent but promising market for intermittent catheters. Growth is propelled by improving healthcare facilities, increasing government investments in healthcare infrastructure, and a gradual rise in awareness about urological conditions and their management. The increasing prevalence of conditions like diabetes, which can lead to neurogenic bladder, further contributes to demand. While the market is currently smaller compared to developed regions, significant potential lies in countries with developing economies and expanding access to specialized medical care, alongside efforts to overcome cultural barriers and enhance patient education.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Intermittent Catheters Market.- Becton, Dickinson and Company (BD)
- Coloplast A/S
- ConvaTec Group Plc
- Cure Medical LLC
- Hollister Incorporated
- B. Braun Melsungen AG
- Teleflex Incorporated
- Wellspect HealthCare (Dentsply Sirona)
- Rusch Inc. (Teleflex)
- Boston Scientific Corporation
- Medline Industries, LP
- Rochester Medical Corporation (C.R. Bard)
- Manfred Sauer GmbH
- CompactCath
- LoFric (Wellspect HealthCare)
- Pennine Healthcare
- Urocare Products Inc.
- Hunter Urology
- PFM Medical
- Mentis Corporation
Frequently Asked Questions
Analyze common user questions about the Intermittent Catheters market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are intermittent catheters primarily used for?
Intermittent catheters are medical devices used for temporary bladder drainage to manage urinary retention or incontinence. They are commonly employed by individuals with neurological conditions like spinal cord injuries or multiple sclerosis, post-operative patients, or those with other urological dysfunctions that prevent complete natural bladder emptying, helping to prevent complications and improve quality of life.
What are the main benefits of using hydrophilic intermittent catheters?
Hydrophilic intermittent catheters offer several key benefits, including significantly reduced friction during insertion and withdrawal due to their lubricious coating, which enhances patient comfort, minimizes urethral trauma, and lowers the risk of long-term complications like strictures. They are also often pre-lubricated or activated with water, making them ready-to-use and highly convenient.
How often should an individual perform intermittent self-catheterization?
The frequency of intermittent self-catheterization is highly individualized and determined by a healthcare professional, typically a urologist or nurse. It depends on factors such as bladder capacity, fluid intake, and the underlying medical condition. Generally, it is performed every 4-6 hours to prevent bladder overdistension and reduce the risk of urinary tract infections, but specific guidance should always come from a clinician.
What are the common risks associated with intermittent catheterization?
While generally safe and effective, common risks associated with intermittent catheterization include urinary tract infections (UTIs), which are the most frequent complication, urethral trauma or irritation, and in rare cases, the development of urethral strictures. Proper hygiene, correct technique, and appropriate catheter selection are crucial for minimizing these risks and ensuring safe practice.
How have recent technological advancements improved intermittent catheters?
Recent technological advancements have significantly improved intermittent catheters by focusing on user comfort and safety. Key innovations include advanced hydrophilic and antimicrobial coatings for reduced friction and infection risk, compact and discreet designs for enhanced convenience and portability, and the use of softer, more biocompatible materials like silicone and polyurethane to minimize urethral irritation. No-touch handling features also improve hygiene and reduce contamination risk.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
- Intermittent Catheters Market Statistics 2025 Analysis By Application (Male Patients, Female Patients, Children), By Type (PVC Intermittent Catheters, Silicone Intermittent Catheters, Red Rubber Intermittent Catheters), and By Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Forecast 2025 to 2032
- Intermittent Catheters Market Size, Share, Trends, & Covid-19 Impact Analysis By Type (Red Rubber Intermittent Catheters, Silicone Intermittent Catheters, PVC Intermittent Catheters), By Application (Children, Female Patients, Male Patients), By Region - North America, Latin America, Europe, Asia Pacific, Middle East, and Africa | In-depth Analysis of all factors and Forecast 2023-2030
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
