Intra-abdominal Pressure Measurement Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430060 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Intra-abdominal Pressure Measurement Devices Market Size
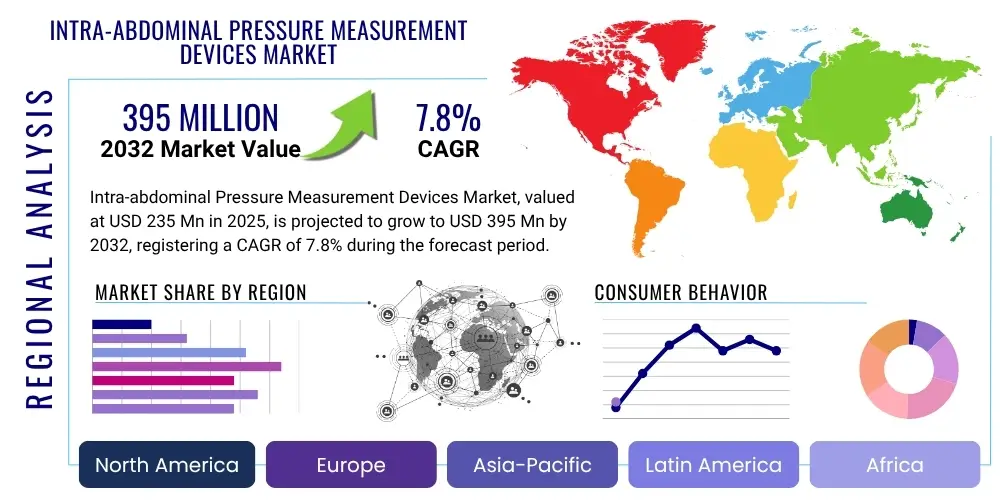
The Intra-abdominal Pressure Measurement Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2025 and 2032. The market is estimated at USD 235 Million in 2025 and is projected to reach USD 395 Million by the end of the forecast period in 2032.
Intra-abdominal Pressure Measurement Devices Market introduction
The Intra-abdominal Pressure (IAP) Measurement Devices Market encompasses a range of medical instruments designed to accurately assess pressure within the abdominal cavity. These devices are crucial for diagnosing and managing conditions such as Intra-abdominal Hypertension (IAH) and Abdominal Compartment Syndrome (ACS), which can lead to severe organ dysfunction and mortality if untreated. Products range from invasive methods, primarily utilizing transducer-tipped catheters inserted into the bladder or stomach, to emerging non-invasive techniques. The primary applications for these devices are found in critical care units, operating rooms, and emergency departments, serving patients with trauma, sepsis, burns, or undergoing major abdominal surgery. The benefits include early detection of elevated IAP, enabling timely intervention, improving patient outcomes, and reducing healthcare costs associated with prolonged complications. Key driving factors for market expansion include the increasing incidence of critical illnesses requiring IAP monitoring, growing awareness among healthcare professionals regarding the importance of IAP management, and continuous technological advancements aimed at improving accuracy and ease of use.
Intra-abdominal Pressure Measurement Devices Market Executive Summary
The Intra-abdominal Pressure Measurement Devices Market is experiencing robust growth driven by advancements in critical care medicine and a heightened understanding of IAP related morbidities. Business trends indicate a focus on product innovation, with manufacturers investing in developing more user-friendly, accurate, and integrated solutions, including wireless capabilities and enhanced data visualization. Mergers and acquisitions are also playing a role in consolidating market share and expanding product portfolios. Regionally, North America and Europe continue to be significant revenue generators due to established healthcare infrastructures and high awareness, while the Asia Pacific region is poised for substantial growth, propelled by increasing healthcare expenditure, a rising prevalence of chronic diseases, and improving access to advanced medical technologies. Segment-wise, the market sees continued dominance of invasive measurement techniques, particularly bladder pressure measurement, due to its reliability and widespread adoption, although there is a gradual shift towards less invasive or non-invasive options. The hospital segment remains the largest end-user, with a growing demand from specialized critical care and surgical units.
AI Impact Analysis on Intra-abdominal Pressure Measurement Devices Market
Users frequently inquire about AI's potential to revolutionize IAP monitoring by enhancing diagnostic accuracy, enabling predictive analytics for patient deterioration, and streamlining clinical workflows. They are keen to understand how AI can move beyond simple data capture to offer actionable insights, integrate with existing hospital information systems, and support personalized treatment protocols. Concerns often revolve around data privacy, regulatory hurdles for AI-powered medical devices, and the need for robust validation studies to ensure reliability and minimize bias. Expectations include AI-driven alerts for impending IAH/ACS, optimization of fluid management strategies, and improved resource allocation in critical care settings, ultimately aiming for better patient outcomes with reduced clinician workload.
- AI can enable real-time analysis of IAP data alongside other physiological parameters, providing a more comprehensive patient status.
- Predictive algorithms powered by AI can forecast the risk of Intra-abdominal Hypertension (IAH) or Abdominal Compartment Syndrome (ACS) development.
- AI assists in optimizing treatment protocols, suggesting individualized fluid management and therapeutic interventions based on patient data.
- Integration of AI with electronic health records (EHR) can automate data logging, reduce manual errors, and improve data accessibility for clinicians.
- Machine learning models can identify subtle patterns in IAP fluctuations that may indicate critical changes, enhancing early detection.
- AI-driven decision support tools can guide less experienced healthcare providers in managing complex IAP scenarios.
DRO & Impact Forces Of Intra-abdominal Pressure Measurement Devices Market
The Intra-abdominal Pressure Measurement Devices Market is primarily driven by the escalating prevalence of conditions such as sepsis, trauma, severe burns, and major abdominal surgeries, all of which necessitate diligent IAP monitoring to prevent severe complications like Abdominal Compartment Syndrome. Technological advancements, particularly in developing more accurate, less invasive, and user-friendly devices, also significantly propel market growth by improving clinical adoption and patient comfort. Growing awareness among healthcare professionals about the critical role of IAP monitoring in improving patient outcomes further stimulates demand. However, the market faces restraints such as the relatively high cost of advanced IAP measurement systems, which can limit adoption in resource-constrained settings, alongside a lack of standardized protocols for IAP measurement and management across all healthcare institutions. Opportunities for market expansion exist in emerging economies with improving healthcare infrastructure and rising medical tourism, as well as in the development of truly non-invasive monitoring technologies and the integration of IAP devices with advanced patient monitoring platforms. Impact forces are shaped by regulatory approvals, reimbursement policies, and the competitive landscape, pushing manufacturers towards innovation while balancing affordability and clinical efficacy.
Segmentation Analysis
The Intra-abdominal Pressure Measurement Devices Market is broadly segmented based on product type, application, and end-user, providing a granular view of market dynamics and growth opportunities. Product types typically include invasive devices like fluid-filled catheter systems and transducer-tipped catheters, which form the core of current market offerings, alongside a growing segment of potentially non-invasive or minimally invasive solutions under development. Applications span across diverse medical fields such as critical care, general surgery, trauma and emergency care, where real-time IAP monitoring is essential for patient management. The end-user segment primarily comprises hospitals, including intensive care units and operating rooms, followed by specialized clinics and ambulatory surgical centers, each demonstrating unique demand patterns influenced by patient volume and specific procedural needs.
- By Product Type
- Invasive Devices
- Transducer-Based Systems
- Foley Catheter-Based Systems
- Intragastric Catheters
- Non-Invasive Devices (Emerging)
- Invasive Devices
- By Application
- Critical Care Units (ICUs)
- General Surgery
- Trauma and Emergency Care
- Burn Units
- Other Applications
- By End-User
- Hospitals
- Ambulatory Surgical Centers
- Specialized Clinics
Value Chain Analysis For Intra-abdominal Pressure Measurement Devices Market
The value chain for Intra-abdominal Pressure Measurement Devices begins with upstream activities involving research and development, raw material sourcing, and component manufacturing. Key suppliers provide specialized sensors, transducers, catheters, and electronic components essential for device functionality. These components are then assembled and integrated by device manufacturers, who focus on design, engineering, and quality control to produce finished IAP monitoring systems. The downstream segment involves marketing, sales, and distribution through various channels. Direct distribution allows manufacturers to engage directly with large hospitals and critical care networks, often involving specialized sales forces and clinical support teams for training and product integration. Indirect distribution, leveraging third-party distributors and wholesalers, extends market reach, particularly in regions where manufacturers lack direct presence, facilitating access to smaller clinics and ambulatory centers. Post-sales services, including maintenance, calibration, and technical support, are also critical components of the value chain, ensuring device longevity and performance.
Intra-abdominal Pressure Measurement Devices Market Potential Customers
The primary end-users and buyers of Intra-abdominal Pressure Measurement Devices are healthcare institutions that manage critically ill patients or those undergoing significant surgical procedures. Hospitals represent the largest customer segment, specifically their Intensive Care Units (ICUs), Surgical Departments, Trauma Centers, and Emergency Rooms, where the need for continuous and accurate IAP monitoring is paramount for patient assessment and intervention. Critical care physicians, surgeons, anesthesiologists, and nurses are the key decision-makers and users within these settings. Additionally, specialized clinics focusing on gastroenterology or surgical recovery may also utilize these devices for specific patient cohorts. The increasing emphasis on evidence-based medicine and protocol-driven care in critical settings further solidifies these institutions as core potential customers, as they seek solutions that provide reliable data to inform clinical decisions and improve patient outcomes.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 235 Million |
| Market Forecast in 2032 | USD 395 Million |
| Growth Rate | 7.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Cousin Biotech, Potts Medical, Spiegelberg GmbH & Co. KG, Becton, Dickinson and Company (BD), Hollistor Inc., ConvaTec Group Plc, Medtronic plc, Teleflex Incorporated, Getinge AB, Cardinal Health Inc., Smiths Medical (now ICU Medical), Philips Healthcare, Dragerwerk AG & Co. KGaA, Hamilton Medical AG, Integra LifeSciences Holdings Corporation, Edwards Lifesciences Corporation, Baxter International Inc., GE Healthcare, Mindray Medical International Limited, Advanced Medical Solutions Group plc |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Intra-abdominal Pressure Measurement Devices Market Key Technology Landscape
The technology landscape for Intra-abdominal Pressure Measurement Devices is characterized by a continuous drive towards enhanced accuracy, ease of use, and integration with broader patient monitoring ecosystems. Currently, the market is dominated by invasive techniques, primarily utilizing bladder pressure measurement via Foley catheters integrated with external pressure transducers. These transducer-based systems convert mechanical pressure into electrical signals for digital display and recording, offering reliable and real-time data. Innovations are focusing on miniaturization of sensors, improving catheter designs for reduced patient discomfort and infection risk, and enhancing connectivity options such as wireless data transmission to central monitoring stations or electronic health records. There is also significant research and development into non-invasive or minimally invasive methods, including techniques leveraging bioimpedance or ultrasound, which promise to overcome the limitations of invasive procedures, such as infection risk and patient compliance, thereby expanding the applicability of IAP monitoring to a wider range of clinical scenarios and potentially home care settings. Digital platforms for data visualization, trending, and alarm management are becoming standard, providing clinicians with more comprehensive insights.
Regional Highlights
- North America: Dominates the market due to advanced healthcare infrastructure, high awareness of IAH/ACS, significant healthcare expenditure, and the presence of key market players and research institutions. The region benefits from robust reimbursement policies for critical care interventions.
- Europe: A mature market with strong growth driven by an aging population, increasing incidence of chronic diseases, and well-established clinical guidelines for IAP management. Countries like Germany, France, and the UK are key contributors, focusing on innovative device adoption and high-quality patient care.
- Asia Pacific (APAC): Expected to exhibit the highest growth rate, fueled by improving healthcare access, rising disposable incomes, rapid expansion of hospital infrastructure, and increasing awareness of advanced medical technologies. Emerging economies like China and India present substantial untapped market potential.
- Latin America: Demonstrates steady growth, supported by governmental initiatives to improve healthcare facilities and increasing investment in medical technologies. Brazil and Mexico are leading countries in the region for market adoption and expansion.
- Middle East and Africa (MEA): Shows gradual but promising growth, particularly in Gulf Cooperation Council (GCC) countries, driven by increasing healthcare spending, modernization of hospitals, and a growing demand for specialized medical devices for critical care and surgical applications.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Intra-abdominal Pressure Measurement Devices Market.- Cousin Biotech
- Potts Medical
- Spiegelberg GmbH & Co. KG
- Becton, Dickinson and Company (BD)
- Hollistor Inc.
- ConvaTec Group Plc
- Medtronic plc
- Teleflex Incorporated
- Getinge AB
- Cardinal Health Inc.
- Smiths Medical (now ICU Medical)
- Philips Healthcare
- Dragerwerk AG & Co. KGaA
- Hamilton Medical AG
- Integra LifeSciences Holdings Corporation
- Edwards Lifesciences Corporation
- Baxter International Inc.
- GE Healthcare
- Mindray Medical International Limited
- Advanced Medical Solutions Group plc
Frequently Asked Questions
What is Intra-abdominal Pressure Measurement and why is it important?
Intra-abdominal Pressure (IAP) measurement quantifies the pressure within the abdominal cavity. It is crucial for diagnosing and managing conditions like Intra-abdominal Hypertension (IAH) and Abdominal Compartment Syndrome (ACS), which can severely impact organ function and patient survival if not promptly addressed.
What are the primary types of IAP measurement devices available?
The primary types of IAP measurement devices include invasive systems, predominantly using fluid-filled catheters or transducer-tipped catheters inserted into the bladder or stomach. Non-invasive methods are under development, aiming to provide less intrusive monitoring solutions.
Which medical conditions necessitate IAP monitoring?
IAP monitoring is typically required for critically ill patients suffering from severe trauma, sepsis, burns, acute pancreatitis, or those who have undergone major abdominal surgery, to prevent and manage IAH and ACS.
What factors are driving the growth of the IAP measurement devices market?
Market growth is driven by the increasing incidence of critical illnesses, continuous technological advancements leading to more accurate and user-friendly devices, and rising awareness among healthcare professionals about the importance of IAP management for improved patient outcomes.
How is AI impacting the future of IAP measurement?
AI is expected to enhance IAP measurement by providing real-time data analysis, predictive insights for early detection of complications, personalized treatment recommendations, and seamless integration with electronic health records, ultimately optimizing patient care and workflow efficiency.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager