Leadless Pacemakers Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427866 | Date : Oct, 2025 | Pages : 242 | Region : Global | Publisher : MRU
Leadless Pacemakers Market Size
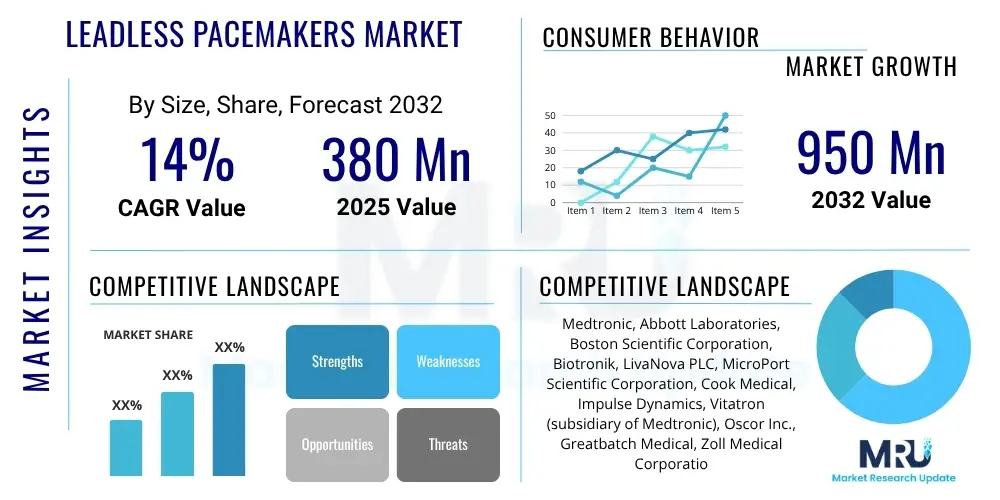
The Leadless Pacemakers Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 14.0% between 2025 and 2032. The market is estimated at USD 380 Million in 2025 and is projected to reach USD 950 Million by the end of the forecast period in 2032.
Leadless Pacemakers Market introduction
The Leadless Pacemakers Market represents a significant advancement in cardiovascular medicine, offering a revolutionary alternative to conventional transvenous pacing systems. Unlike traditional pacemakers that require leads to deliver electrical impulses to the heart, leadless devices are miniature, self-contained units directly implanted into the right ventricle. This innovative design eliminates common complications associated with leads, such as infection, fracture, or dislodgement, thereby enhancing patient safety and long-term outcomes. The introduction of leadless technology addresses critical unmet needs in a substantial patient population suffering from symptomatic bradycardia, a condition characterized by an abnormally slow heart rate that can lead to dizziness, fatigue, and syncope. These devices are particularly beneficial for elderly patients, individuals with venous access issues, or those prone to lead-related complications, offering a less invasive and more comfortable solution for managing their cardiac health.
Major applications for leadless pacemakers primarily include the treatment of bradycardia, especially in patients who are at high risk for complications from traditional transvenous leads, or those requiring single-chamber ventricular pacing. The benefits of these devices extend beyond reducing lead-related issues to include improved patient quality of life due to the smaller incision site, faster recovery times, and often, an invisible implant. Key driving factors propelling market growth include the continuously expanding global geriatric population, which is inherently more susceptible to cardiac arrhythmias, a rising global prevalence of chronic cardiovascular diseases requiring pacing interventions, and a strong preference among both patients and healthcare providers for minimally invasive procedures that promise reduced procedural risks and enhanced cosmetic outcomes. Ongoing technological advancements focused on improving battery longevity, miniaturization, and enhancing sensing capabilities are further solidifying the market's trajectory towards sustained expansion.
Leadless Pacemakers Market Executive Summary
The Leadless Pacemakers Market is characterized by dynamic business trends, marked by significant investment in research and development aimed at further miniaturization and enhanced functionalities. Manufacturers are intensely focused on expanding the clinical indications for these devices, exploring avenues for multi-chamber pacing solutions that could address a broader spectrum of cardiac conditions beyond single-chamber bradycardia. Remote monitoring capabilities, often integrated with AI-driven analytics, are becoming a standard offering, enabling clinicians to track patient health and device performance proactively, leading to more personalized care and early detection of potential issues. Strategic collaborations between medical device companies and digital health platforms are also a prominent trend, aiming to create comprehensive ecosystem solutions for cardiac rhythm management, ultimately improving patient engagement and overall clinical efficiency within the healthcare landscape.
Regional trends indicate North America and Europe as dominant markets, primarily due to advanced healthcare infrastructure, higher adoption rates of innovative medical technologies, and favorable reimbursement policies. However, the Asia Pacific region is rapidly emerging as a high-growth market, driven by an increasing awareness of leadless technologies, improving healthcare access, and a burgeoning aging population coupled with a rising incidence of cardiovascular diseases. Within segments, single-chamber leadless pacemakers currently constitute the majority of the market, but there is substantial research and development investment directed towards multi-chamber leadless systems, which are anticipated to unlock significant future growth opportunities by addressing a wider range of complex cardiac conditions. The market is also witnessing a gradual shift towards ambulatory surgical centers for implantation procedures, reflecting the minimally invasive nature of these devices and the desire for cost-effective treatment settings.
AI Impact Analysis on Leadless Pacemakers Market
User inquiries regarding the impact of Artificial Intelligence on the Leadless Pacemakers Market frequently revolve around how AI can enhance the efficacy, safety, and operational aspects of these devices. Common questions explore AI's role in improving diagnostic accuracy for cardiac arrhythmias, enabling personalized pacing therapies, optimizing device performance and longevity through predictive analytics, and refining remote monitoring capabilities. Users are also keen on understanding how AI might contribute to the development of next-generation leadless pacemakers, particularly in areas like autonomous decision-making for pacing adjustments, early detection of potential complications, and streamlining clinical workflows. There is also interest in the ethical implications and data privacy concerns associated with AI integration in such critical medical devices, alongside expectations for AI to make these devices more accessible and cost-effective over time. The consensus points towards AI being a transformative force, enabling more intelligent, adaptive, and patient-centric cardiac rhythm management solutions.
- AI algorithms can analyze vast amounts of real-time cardiac data collected by leadless pacemakers, identifying subtle patterns indicative of impending arrhythmias or device malfunctions, thereby enabling proactive intervention and improving patient safety.
- Personalized pacing therapy, tailored to individual patient physiological responses and lifestyle, can be achieved through AI by dynamically adjusting pacing parameters based on activity levels, heart rate variability, and other biometric inputs, optimizing cardiac output and patient comfort.
- Predictive maintenance capabilities, leveraging AI to forecast battery depletion or component wear, can extend the operational life of leadless pacemakers and allow for timely replacements, minimizing unexpected device failures and associated health risks.
- AI-powered remote monitoring systems can significantly reduce the burden on healthcare systems by intelligently filtering clinically relevant events from routine data, alerting clinicians only to critical changes, and facilitating more efficient patient management from a distance.
- Enhancement of diagnostic capabilities through AI allows for more accurate and rapid identification of complex cardiac conditions that might otherwise be missed, leading to earlier treatment and improved long-term outcomes for patients with various forms of bradycardia or heart block.
- Streamlined clinical trial processes and accelerated product development can benefit from AI by analyzing large datasets to identify optimal design parameters, predict device performance, and simulate clinical scenarios, bringing advanced leadless technologies to market more rapidly and efficiently.
DRO & Impact Forces Of Leadless Pacemakers Market
The Leadless Pacemakers Market is significantly propelled by several robust drivers, fundamentally rooted in demographic shifts and technological advancements. The global aging population is a primary catalyst, as the elderly are disproportionately affected by cardiac arrhythmias, including bradycardia, necessitating pacing interventions. Concurrently, the increasing worldwide prevalence of chronic cardiovascular diseases, such as atrial fibrillation and heart failure, contributes substantially to the demand for effective and minimally invasive rhythm management solutions. Patient and physician preference for less invasive procedures plays a pivotal role, driven by the promise of reduced recovery times, lower infection risks, and improved cosmetic outcomes compared to traditional pacemakers. Furthermore, continuous innovation in battery technology, miniaturization, and advanced sensing capabilities are enhancing device longevity and efficacy, making leadless pacemakers a more attractive option.
However, the market also faces considerable restraints that temper its growth trajectory. The high upfront cost of leadless pacemakers remains a significant barrier, especially in emerging economies and for healthcare systems with constrained budgets, limiting broader adoption. Complex and stringent regulatory approval processes, particularly in major markets such as the United States and Europe, can delay market entry for new devices and increase development costs, thereby impacting innovation cycles. Limited reimbursement policies in some regions or for specific patient profiles can further hinder market penetration, as healthcare providers may be reluctant to invest in procedures that are not adequately covered. Additionally, the specialized technical expertise required for leadless pacemaker implantation, coupled with the relatively steep learning curve for cardiologists, contributes to a shortage of skilled professionals, which can slow down adoption rates in various geographical areas.
Despite these challenges, substantial opportunities exist for market expansion and innovation. The development of multi-chamber leadless pacemakers, capable of pacing both the atrium and ventricle, holds immense potential to expand the addressable patient population, moving beyond the current single-chamber indications. Emerging markets, with their rapidly developing healthcare infrastructure and increasing affordability, present significant untapped growth prospects for leadless technology. Integration with advanced digital health platforms, remote patient monitoring systems, and artificial intelligence offers avenues for enhancing device intelligence, patient management, and overall clinical efficiency, creating a comprehensive ecosystem of care. Moreover, ongoing research into novel energy harvesting techniques and advanced biocompatible materials could further improve device longevity and reduce the need for replacement procedures, strengthening the long-term value proposition of leadless pacemakers and potentially lowering lifetime costs.
Segmentation Analysis
The Leadless Pacemakers Market is comprehensively segmented to provide a detailed understanding of its diverse components and growth dynamics. These segmentations allow for granular analysis of market trends, identification of key growth areas, and strategic planning for market participants. The primary segmentation categories typically include product type, end-user, and geographical region, each revealing distinct patterns and opportunities within the evolving landscape of cardiac rhythm management. Understanding these segments is crucial for stakeholders to tailor their product offerings, marketing strategies, and distribution channels to meet the specific demands of different market niches and patient populations effectively. The continuous innovation in leadless technology influences these segments, particularly with the anticipated introduction of more advanced product types that will further refine the market structure and competitive dynamics.
- Product Type: This segment is primarily categorized into Single-Chamber Leadless Pacemakers, which currently dominate the market due to their established clinical efficacy and regulatory approvals, and Multi-Chamber Leadless Pacemakers, which represent a significant future growth avenue as research and development efforts aim to overcome technical complexities for broader indications.
- End-User: Key end-users include Hospitals, which serve as the primary sites for complex cardiac procedures and post-operative care, Cardiac Centers, often specialized institutions focused on cardiovascular health, and a growing number of Ambulatory Surgical Centers, which are increasingly equipped to handle minimally invasive procedures for eligible patients seeking more convenient and cost-effective care settings.
- Region: The market is geographically segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa, each exhibiting varying levels of market maturity, technological adoption rates, healthcare spending, and prevalence of cardiovascular diseases, influencing their respective growth trajectories.
Value Chain Analysis For Leadless Pacemakers Market
The value chain for the Leadless Pacemakers Market is intricate, involving a series of sequential activities that transform raw materials into a finished product delivered to the end-user. It begins with upstream activities focused on the procurement of specialized components and materials, requiring stringent quality control and innovation in materials science. This phase involves sourcing high-grade biocompatible materials, advanced battery components, miniaturized electronic circuits, and sophisticated sensors from specialized suppliers. Manufacturers in this segment face challenges related to supply chain resilience, cost management, and adherence to evolving regulatory standards for medical device components, underscoring the importance of robust supplier relationships and internal quality assurance processes.
The midstream segment of the value chain is dominated by the manufacturing and assembly of the leadless pacemakers. This stage demands highly specialized engineering expertise, precision manufacturing techniques, and sterile production environments. Companies invest heavily in research and development to innovate device design, improve battery life, enhance pacing algorithms, and ensure the long-term reliability and safety of the implanted device. Post-manufacturing, rigorous testing, quality assurance, and regulatory compliance are paramount before the devices can be released for distribution. This phase represents a significant capital expenditure for market players, requiring substantial investment in advanced manufacturing facilities and a highly skilled workforce, contributing to the overall cost structure of these sophisticated medical devices.
Downstream activities encompass the distribution, sales, and post-sale support. Products typically move through both direct and indirect distribution channels. Direct sales involve manufacturers engaging directly with hospitals, cardiac centers, and electrophysiology laboratories, offering personalized sales support, training, and clinical education to medical professionals. Indirect channels involve partnering with specialized medical device distributors who have established networks and logistics capabilities, particularly in regions where manufacturers lack a direct presence. The distribution network must ensure efficient delivery while maintaining the integrity and sterility of the devices. Post-sale, ongoing clinical support, patient monitoring, device maintenance, and periodic software updates are critical components, ensuring optimal patient outcomes and fostering long-term relationships with healthcare providers and patients, thereby closing the loop of the value chain.
Leadless Pacemakers Market Potential Customers
The primary potential customers and end-users of leadless pacemakers are multifaceted, encompassing both the medical professionals who implant and manage these devices and the patients who receive them. At the forefront are cardiologists and electrophysiologists, who are the key decision-makers and prescribers of cardiac rhythm management therapies. These specialists evaluate patient eligibility, perform the implantation procedures, and manage post-operative care, requiring extensive training and adherence to specific clinical guidelines. Hospitals and specialized cardiac centers serve as crucial institutional customers, as they are the primary settings where these advanced procedures are performed, investing in the necessary equipment, facilities, and personnel to support leadless pacemaker implantation programs. Their purchasing decisions are influenced by clinical efficacy, patient outcomes, cost-effectiveness, and the availability of comprehensive support from manufacturers, making them central to market adoption.
In addition to hospitals and cardiologists, ambulatory surgical centers (ASCs) are emerging as significant potential customers, particularly as leadless pacemaker implantation procedures become more standardized and less invasive. ASCs offer a cost-effective and convenient alternative to traditional hospital settings for eligible patients, attracting providers looking to optimize operational efficiency and patients seeking a more streamlined experience. This segment's growth is driven by trends towards outpatient care and favorable reimbursement policies for minimally invasive procedures. Furthermore, general practitioners and referring physicians also play an indirect but vital role as they are often the first point of contact for patients experiencing bradycardia symptoms, guiding them towards specialists who can recommend leadless pacemakers, thus influencing the initial patient funnel for these advanced devices.
Ultimately, the patients themselves represent the final end-users, with specific demographic and clinical profiles making them ideal candidates for leadless technology. These include individuals suffering from symptomatic bradycardia who require permanent pacing, particularly elderly patients who may be at higher risk for complications associated with traditional transvenous leads. Patients with limited venous access, a history of lead-related complications (such as infections or fractures), or those who prioritize a cosmetically discreet and minimally invasive solution are also strong potential customers. The increasing awareness among the general public about innovative medical technologies, coupled with the desire for improved quality of life and reduced procedural risks, further drives patient-initiated inquiries and preferences for leadless pacemakers, influencing the demand side of the market significantly.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 380 Million |
| Market Forecast in 2032 | USD 950 Million |
| Growth Rate | 14.0% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, Abbott Laboratories, Boston Scientific Corporation, Biotronik, LivaNova PLC, MicroPort Scientific Corporation, Cook Medical, Impulse Dynamics, Vitatron (subsidiary of Medtronic), Oscor Inc., Greatbatch Medical, Zoll Medical Corporation, Sorin Group (now part of LivaNova), CardioFocus Inc., EBR Systems, Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Leadless Pacemakers Market Key Technology Landscape
The technological landscape of the Leadless Pacemakers Market is characterized by continuous innovation aimed at enhancing device performance, reducing size, and improving patient outcomes. Miniaturization stands as a cornerstone technology, enabling the development of self-contained units that can be implanted directly into the heart without the need for transvenous leads. This involves highly specialized microelectronics, compact energy storage solutions, and advanced packaging techniques to ensure biocompatibility and reliability within the cardiovascular system. Advancements in battery technology are critical, focusing on increasing longevity to minimize replacement procedures and ensure sustained pacing therapy over many years. This includes research into novel power sources and energy-efficient circuits that optimize device operation, thereby extending the clinical utility and patient value proposition of leadless devices significantly.
Wireless communication and remote monitoring platforms constitute another pivotal technological area, allowing for the seamless transmission of device performance data and patient physiological parameters to healthcare providers. This technology leverages secure, low-power radiofrequency communication protocols, often integrated with cloud-based analytics, to facilitate continuous oversight and proactive management of patients. The integration of advanced sensing capabilities, utilizing sophisticated algorithms, enables leadless pacemakers to accurately detect intrinsic cardiac activity and deliver therapy only when needed, optimizing energy consumption and physiological pacing. Furthermore, biocompatible materials science is crucial for ensuring the long-term safety and efficacy of these implanted devices, preventing adverse tissue reactions and promoting stable integration within the cardiac environment, which is paramount for patient acceptance and device longevity.
Looking ahead, the technological landscape is increasingly embracing Artificial Intelligence (AI) and machine learning algorithms. AI is being deployed to interpret complex cardiac signals, provide personalized pacing adjustments based on individual patient needs, and predict potential cardiac events or device malfunctions, thereby transforming reactive care into proactive management. Development efforts are also focused on creating multi-chamber leadless pacing systems that can mimic the functions of conventional dual-chamber pacemakers, significantly broadening the therapeutic indications and patient population that can benefit from leadless technology. This involves intricate synchronization algorithms and advanced power management to support multiple pacing sites within a miniature form factor. These technological breakthroughs are not only enhancing the current capabilities of leadless pacemakers but are also paving the way for future generations of smart, adaptive, and fully integrated cardiac rhythm management solutions that promise to revolutionize cardiovascular care.
Regional Highlights
- North America: This region is a dominant force in the Leadless Pacemakers Market, primarily driven by a robust healthcare infrastructure, high awareness and rapid adoption of advanced medical technologies, and favorable reimbursement policies for innovative cardiac devices. The United States, in particular, leads in research and development, clinical trials, and market penetration, supported by a significant aging population and a high prevalence of cardiovascular diseases, alongside extensive investment in specialized cardiology centers and highly skilled electrophysiologists.
- Europe: The European market demonstrates substantial growth, characterized by strong regulatory support for medical innovations and a well-established network of healthcare systems. Countries such as Germany, France, and the UK are key contributors, benefiting from an increasing elderly population, a high incidence of bradycardia, and a growing emphasis on minimally invasive procedures. However, market adoption can vary based on national healthcare policies, reimbursement structures, and patient access to specialized cardiac care, though overall trends lean towards increased acceptance of leadless technology.
- Asia Pacific (APAC): The APAC region is poised for remarkable growth, driven by rapidly improving healthcare infrastructure, increasing disposable incomes, and a burgeoning patient pool susceptible to cardiovascular diseases, especially in densely populated countries like China, India, and Japan. While adoption rates have historically been lower due to cost sensitivity and regulatory complexities, increasing awareness, government initiatives to modernize healthcare, and a growing preference for advanced Western medical technologies are fueling significant market expansion and investment.
- Latin America: The market in Latin America is in an nascent stage but exhibits promising growth potential, influenced by expanding healthcare access, increasing healthcare expenditures, and a rising prevalence of cardiovascular diseases. Countries such as Brazil, Mexico, and Argentina are gradually adopting leadless pacemakers, albeit at a slower pace due to economic constraints, fragmented healthcare systems, and less developed reimbursement frameworks. However, increasing medical tourism and a growing demand for advanced treatments are contributing to gradual market penetration.
- Middle East and Africa (MEA): This region presents a mixed but developing landscape for leadless pacemakers. Growth is primarily concentrated in the wealthier Gulf Cooperation Council (GCC) countries, which boast advanced healthcare facilities, high per capita healthcare spending, and a readiness to adopt cutting-edge medical technologies. In contrast, parts of Africa face significant challenges due to limited healthcare infrastructure, lower awareness, and economic barriers, yet there is a gradual increase in demand as healthcare systems evolve and access to specialized cardiac care improves.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Leadless Pacemakers Market.- Medtronic
- Abbott Laboratories
- Boston Scientific Corporation
- Biotronik
- LivaNova PLC
- MicroPort Scientific Corporation
- Cook Medical
- Impulse Dynamics
- Vitatron (subsidiary of Medtronic)
- Oscor Inc.
- Greatbatch Medical
- Zoll Medical Corporation
- Sorin Group (now part of LivaNova)
- CardioFocus Inc.
- EBR Systems, Inc.
- Pacesetter, Inc. (now part of Abbott)
- Lepu Medical Technology
- Terumo Corporation
Frequently Asked Questions
What are the primary advantages of leadless pacemakers compared to traditional pacemakers?
Leadless pacemakers offer several significant advantages, most notably the elimination of transvenous leads, which are a common source of complications such as infection, lead fracture, or dislodgement. Their miniature, self-contained design allows for direct implantation into the heart, reducing the need for a surgical pocket and external scarring, leading to improved cosmetic outcomes and enhanced patient comfort. This also results in fewer post-implantation restrictions, a faster recovery time, and a reduced risk of serious complications associated with lead failure, ultimately improving patient quality of life and long-term device reliability for individuals requiring cardiac pacing.
Who is an ideal candidate for a leadless pacemaker?
An ideal candidate for a leadless pacemaker is typically a patient suffering from symptomatic bradycardia who requires single-chamber ventricular pacing. This often includes elderly individuals who are at a higher risk of developing complications from traditional transvenous leads, or those with anatomical challenges such as limited venous access. Patients with a history of lead-related infections, those who prioritize a minimally invasive procedure with fewer visible signs of an implant, or individuals with specific comorbidities that preclude the use of traditional systems, are also excellent candidates for this advanced cardiac pacing technology, providing a safer and more convenient alternative.
What is the expected lifespan of a leadless pacemaker, and how is battery depletion managed?
The expected lifespan of a leadless pacemaker generally ranges from 8 to 13 years, depending on the specific model, device settings, and the patient's physiological pacing requirements. This impressive longevity is achieved through advanced battery technology and energy-efficient designs. When the battery approaches depletion, the device can be retrieved in a minimally invasive procedure, and a new leadless pacemaker can be implanted, often alongside the original device if it is not easily retrievable. Ongoing research into energy harvesting and more durable battery chemistries aims to further extend this lifespan, minimizing the need for replacement procedures and enhancing the overall convenience for patients.
How do leadless pacemakers integrate with remote monitoring technologies?
Leadless pacemakers are increasingly designed with integrated wireless communication capabilities that enable seamless remote monitoring. This technology allows the device to transmit crucial data about its performance, battery status, and the patient's cardiac rhythm directly to healthcare providers through a secure, external home monitor or a compatible smart device. Remote monitoring significantly enhances patient care by facilitating early detection of potential issues, reducing the need for frequent in-person clinic visits, and providing clinicians with continuous insights into the patient's cardiac health, thereby enabling proactive adjustments to pacing therapy and improving overall clinical management efficiency and patient safety without requiring an additional device.
What are the current limitations and future developments expected in the leadless pacemakers market?
While highly advanced, current leadless pacemakers are primarily limited to single-chamber ventricular pacing, restricting their applicability for patients who require dual-chamber or atrial pacing. The relatively high cost compared to traditional pacemakers can also be a barrier to widespread adoption in some healthcare systems. Future developments are expected to address these limitations, with significant research focused on the creation of multi-chamber leadless systems, which would enable pacing in both the atria and ventricles, expanding the treatable patient population. Advancements in miniaturization, AI integration for personalized therapy, extended battery life, and cost reduction through innovative manufacturing processes are also anticipated, aiming to make leadless technology more accessible, versatile, and suitable for an even broader range of cardiac rhythm disorders.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
