Lipid Nanoparticles Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430572 | Date : Nov, 2025 | Pages : 253 | Region : Global | Publisher : MRU
Lipid Nanoparticles Market Size
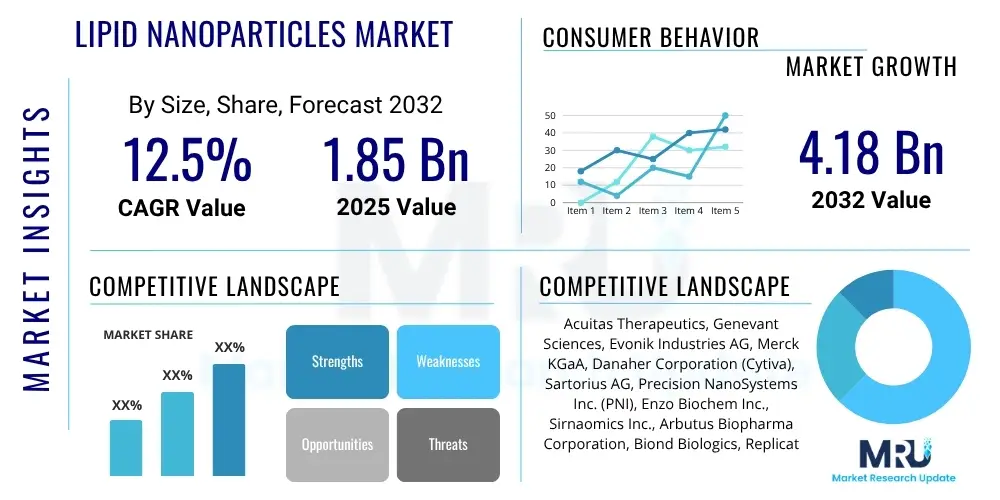
The Lipid Nanoparticles Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2025 and 2032. The market is estimated at USD 1.85 Billion in 2025 and is projected to reach USD 4.18 Billion by the end of the forecast period in 2032.
Lipid Nanoparticles Market introduction
The Lipid Nanoparticles Market is witnessing significant expansion, driven by their pivotal role as advanced drug delivery systems. Lipid nanoparticles (LNPs) are innovative colloidal systems composed of lipids that encapsulate therapeutic agents such as mRNA, DNA, small molecules, and proteins, protecting them from degradation and facilitating targeted cellular uptake. Their primary applications span a broad spectrum, including highly effective mRNA vaccines for infectious diseases, gene therapies for genetic disorders, and novel therapeutics in oncology, offering enhanced drug stability, improved bioavailability, and reduced off-target toxicity. The inherent benefits of LNPs, such as their biocompatibility, biodegradability, and ability to traverse biological barriers, underpin their growing adoption across the pharmaceutical and biotechnology sectors.
The market's rapid growth is further fueled by escalating global investment in biopharmaceutical research and development, particularly in areas requiring precise and efficient nucleic acid delivery. The success of mRNA-based COVID-19 vaccines dramatically accelerated LNP technology maturation and public awareness, paving the way for numerous therapeutic applications. These nanoparticles provide a robust platform for delivering sensitive biomolecules to specific cells or tissues, overcoming many challenges associated with traditional drug administration. As such, LNPs are becoming indispensable tools in the development of next-generation medicines, promising to revolutionize treatment paradigms for a variety of complex diseases.
Lipid Nanoparticles Market Executive Summary
The Lipid Nanoparticles Market is poised for substantial growth, propelled by groundbreaking innovations in gene therapy and mRNA vaccine development. Business trends indicate a surge in strategic collaborations, mergers, and acquisitions aimed at expanding technological capabilities and market reach, with pharmaceutical giants and specialized biotech firms investing heavily in LNP platforms. Regionally, North America and Europe continue to dominate due to robust research infrastructure, significant R&D spending, and supportive regulatory environments, while the Asia Pacific region is emerging as a high-growth market driven by increasing healthcare expenditure and expanding biopharmaceutical manufacturing capacities. Segment-wise, mRNA vaccines and gene therapies are the leading application areas, demonstrating rapid advancements and attracting considerable investment, alongside promising developments in oncology and rare disease treatments.
AI Impact Analysis on Lipid Nanoparticles Market
Users frequently inquire about how Artificial Intelligence (AI) can revolutionize the design, development, and optimization of Lipid Nanoparticles. Common questions highlight expectations for AI to accelerate drug discovery, enhance formulation stability, predict in vivo performance, and streamline manufacturing processes. There is significant interest in AI's role in identifying novel lipid compositions, understanding complex biological interactions, and tailoring LNPs for specific therapeutic targets, addressing challenges related to efficiency, cost, and safety. Concerns often revolve around the validation of AI models, the need for large, high-quality datasets, and ensuring regulatory compliance for AI-driven advancements in LNP technology.
- AI expedites LNP formulation design by screening vast libraries of lipids and excipients, predicting optimal compositions for stability and efficacy.
- Predictive modeling using AI optimizes manufacturing parameters, reducing experimental iterations and scaling up production more efficiently.
- AI algorithms analyze complex biological data to identify optimal LNP characteristics for targeted delivery and reduced off-target effects.
- Drug discovery is accelerated through AI-driven identification of novel therapeutic payloads suitable for LNP encapsulation.
- Quality control and assurance are enhanced by AI-powered analytics monitoring LNP integrity, size distribution, and batch consistency in real-time.
- AI assists in understanding the complex interactions between LNPs and biological systems, improving safety and immunogenicity profiles.
- Personalized medicine benefits from AI by enabling rapid customization of LNP formulations for individual patient needs based on genetic profiles.
DRO & Impact Forces Of Lipid Nanoparticles Market
The Lipid Nanoparticles Market is significantly influenced by a confluence of driving factors, restrictive elements, and burgeoning opportunities that collectively shape its trajectory. Key drivers include the exponential growth in demand for advanced drug delivery systems, particularly for nucleic acid-based therapeutics such as mRNA vaccines and gene therapies, which gained unprecedented traction following the global health crisis. Furthermore, continuous advancements in lipid chemistry and formulation technologies, coupled with increasing R&D investments by pharmaceutical and biotechnology companies, are propelling market expansion. The proven efficacy and safety profile of LNP-based therapeutics in clinical settings further reinforce their adoption across various therapeutic areas, including oncology, infectious diseases, and rare genetic disorders.
Conversely, the market faces several restraints, including the high cost associated with LNP research, development, and manufacturing, which can be prohibitive for smaller biotech firms. The stringent and evolving regulatory landscape for novel drug delivery systems, especially for gene-editing technologies, poses a considerable challenge, requiring extensive clinical trials and robust safety data. Technical complexities in achieving scalable and consistent LNP production, along with intellectual property rights issues, also hinder market growth. Despite these obstacles, the market presents immense opportunities driven by the emergence of personalized medicine, the exploration of LNPs in diagnostic applications, and the potential for non-viral gene delivery platforms. The growing pipeline of LNP-encapsulated therapies targeting a wider array of diseases underscores the significant untapped potential within this innovative field.
The impact forces at play are multi-faceted, encompassing technological advancements that enable more precise targeting and controlled release of therapeutics, regulatory reforms that streamline approval processes for innovative medicines, and economic factors such as healthcare spending and investment in biotechnological infrastructure. The competitive landscape is also a strong impact force, with intense rivalry among established players and emerging startups driving continuous innovation. Global health initiatives and public-private partnerships aimed at addressing unmet medical needs further amplify the market's growth potential. These intertwined forces dictate the pace of LNP adoption and the evolution of its applications across the healthcare ecosystem.
Segmentation Analysis
The Lipid Nanoparticles Market is comprehensively segmented based on various critical parameters including type of lipid, application area, and end-user, providing a detailed understanding of its diverse landscape. This segmentation allows for a nuanced analysis of market dynamics, identifying specific growth drivers and challenges within each category. The distinction by lipid type highlights the varying functional characteristics and suitability for different payloads, while application segmentation showcases the therapeutic breadth of LNPs. End-user categorization reflects the primary consumers of LNP technologies, from research institutions to commercial biopharmaceutical entities, offering insights into market demand and adoption patterns.
- By Type
- Cationic Lipids
- Ionizable Lipids
- Helper Lipids
- PEGylated Lipids
- Others (e.g., Neutral Lipids, Sterols)
- By Application
- mRNA Vaccines
- Gene Therapy (e.g., CRISPR/Cas9 delivery, RNAi)
- Cancer Therapeutics
- Protein Delivery
- Diagnostics
- Other Therapeutic Areas (e.g., Autoimmune Diseases, Infectious Diseases beyond mRNA vaccines)
- By End User
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic and Research Institutes
Value Chain Analysis For Lipid Nanoparticles Market
The value chain for the Lipid Nanoparticles Market is intricate, encompassing various stages from raw material sourcing to end-user delivery, highlighting the interconnectedness of specialized suppliers and service providers. Upstream analysis reveals a critical dependency on suppliers of high-purity lipids (such as ionizable lipids, phospholipids, cholesterol, and PEGylated lipids), as well as other excipients and analytical reagents. These raw materials are fundamental to LNP formulation, and their quality, consistency, and supply chain reliability are paramount for successful therapeutic development. The synthesis and purification of these specialized lipids require advanced chemical manufacturing capabilities, often provided by a select group of niche chemical companies and CDMOs.
Further down the chain, LNP manufacturing and formulation services constitute a crucial midstream segment, where contract development and manufacturing organizations (CDMOs) play a significant role. These organizations provide expertise in LNP encapsulation, optimization, and scale-up using advanced technologies like microfluidics and T-junction mixing. Downstream analysis focuses on the integration of these formulated LNPs into final drug products by pharmaceutical and biotechnology companies. This stage involves preclinical and clinical development, regulatory approval processes, and large-scale commercial manufacturing, culminating in the distribution and administration of LNP-based therapeutics to patients globally. The effectiveness of the overall value chain relies on seamless collaboration and stringent quality control at each transition point.
Distribution channels for LNP-based products are typically direct or indirect. Direct channels involve pharmaceutical and biotech companies selling their approved LNP therapeutics directly to hospitals, clinics, and government health programs. Indirect channels often involve partnerships with specialized distributors, wholesalers, and third-party logistics providers to ensure widespread market access and efficient cold chain management, particularly for temperature-sensitive products like mRNA vaccines. Both direct and indirect models are essential to reach diverse patient populations and integrate LNP therapies into established healthcare systems, ensuring timely and effective delivery to the ultimate beneficiaries.
Lipid Nanoparticles Market Potential Customers
The Lipid Nanoparticles Market serves a diverse range of potential customers, primarily comprising entities within the biopharmaceutical and research sectors that require advanced drug delivery solutions. The primary end-users and buyers of LNP technologies and products are pharmaceutical companies, which are actively engaged in developing and commercializing a broad spectrum of therapeutics for various indications, including oncology, infectious diseases, and genetic disorders. These companies leverage LNPs to enhance the efficacy, safety, and targeting capabilities of their drug candidates, addressing limitations of traditional delivery methods and expanding their product pipelines.
Biotechnology companies also represent a significant customer segment. These firms, often focused on cutting-edge research in gene editing, RNA therapeutics, and novel vaccine platforms, are early adopters and key innovators in LNP application. They seek LNP expertise and ready-to-use formulations to bring their innovative discoveries from the lab to clinical trials more efficiently. Contract Research Organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs) are another vital customer group, as they provide specialized services to pharmaceutical and biotech companies, including LNP formulation, characterization, and scale-up, acting as intermediaries and solution providers within the market.
Furthermore, academic and research institutions globally constitute a foundational customer base. These institutions conduct fundamental research into LNP mechanisms, explore new lipid chemistries, and identify novel therapeutic applications, often relying on specialized LNP components and custom formulation services. Government health agencies and non-profit organizations, particularly those involved in global health initiatives and vaccine development, also act as significant buyers, especially in the context of pandemic preparedness and public health interventions requiring rapid deployment of LNP-enabled vaccines or treatments. This broad customer base underscores the pervasive and critical role of LNPs across the entire biopharmaceutical ecosystem.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 1.85 Billion |
| Market Forecast in 2032 | USD 4.18 Billion |
| Growth Rate | 12.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Acuitas Therapeutics, Genevant Sciences, Evonik Industries AG, Merck KGaA, Danaher Corporation (Cytiva), Sartorius AG, Precision NanoSystems Inc. (PNI), Enzo Biochem Inc., Sirnaomics Inc., Arbutus Biopharma Corporation, Biond Biologics, Replicate Bioscience, CureVac N.V., BioNTech SE, Moderna Inc., Translate Bio (Sanofi), Alnylam Pharmaceuticals, Roche, Pfizer, AstraZeneca |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Lipid Nanoparticles Market Key Technology Landscape
The technology landscape for the Lipid Nanoparticles Market is characterized by a blend of sophisticated formulation methods and advanced analytical techniques essential for producing stable, efficacious, and reproducible LNPs. Key manufacturing technologies revolve around microfluidics, a precision engineering approach that allows for rapid and controlled mixing of lipid and aqueous phases in microchannels. This method enables the reproducible formation of LNPs with narrow size distributions, critical for consistent drug delivery and reduced immunogenicity. Other prominent techniques include T-junction mixing, which offers scalability for larger batch production, and ethanol injection methods, often used in early-stage research and development due to its simplicity.
Beyond formulation, specialized encapsulation technologies are crucial for effectively loading therapeutic payloads, particularly fragile nucleic acids, into the lipid core while maintaining their integrity. These technologies ensure high encapsulation efficiency and protect the payload from enzymatic degradation in biological environments. Parallel advancements in high-pressure homogenization and extrusion methods are employed for refining LNP size and uniformity. The entire process from raw material preparation to final LNP purification demands rigorous control to meet pharmaceutical standards, leading to the continuous development of automated and closed-system manufacturing platforms to enhance sterility and scalability.
Analytical technologies form another critical pillar of the LNP technology landscape, indispensable for characterization and quality control. Techniques such as Dynamic Light Scattering (DLS) are routinely used to determine particle size and polydispersity, while Transmission Electron Microscopy (TEM) provides visual confirmation of LNP morphology. High-Performance Liquid Chromatography (HPLC) and Liquid Chromatography–Mass Spectrometry (LC–MS) are vital for lipid quantification and purity assessment, and specialized assays measure encapsulation efficiency and payload integrity. These advanced analytical tools are paramount for ensuring the quality, safety, and performance of LNP-based therapeutics throughout their lifecycle, from development to commercial production.
Regional Highlights
- North America: Dominates the Lipid Nanoparticles Market due to extensive research and development activities, significant investments in biotechnology, and the presence of numerous key pharmaceutical and biotech companies. The United States, in particular, leads in innovation, funding for gene therapies and mRNA vaccines, and a favorable regulatory environment for novel drug delivery systems.
- Europe: Holds a substantial share of the market, driven by a strong academic research base, established pharmaceutical industry, and increasing government support for advanced therapies. Countries like Germany, the UK, and France are at the forefront of LNP development, benefiting from a robust healthcare infrastructure and collaborative research networks.
- Asia Pacific (APAC): Expected to exhibit the highest growth rate during the forecast period. This growth is attributed to rising healthcare expenditure, improving R&D capabilities, increasing prevalence of chronic diseases, and a growing focus on biosimilars and generics. China, Japan, and India are key contributors, with emerging markets investing heavily in biopharmaceutical manufacturing and research.
- Latin America: Showing nascent growth in the LNP market, primarily driven by increasing awareness of advanced therapies, improving healthcare access, and expanding pharmaceutical markets in countries like Brazil and Mexico. However, market penetration is slower compared to developed regions due to economic and infrastructure challenges.
- Middle East and Africa (MEA): Represents the smallest but a developing segment of the LNP market. Growth is primarily propelled by strategic investments in healthcare infrastructure, increasing collaborations with international pharmaceutical companies, and efforts to diversify economies from oil dependence. Challenges include limited R&D capabilities and regulatory hurdles.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Lipid Nanoparticles Market.- Acuitas Therapeutics
- Genevant Sciences
- Evonik Industries AG
- Merck KGaA
- Danaher Corporation (Cytiva)
- Sartorius AG
- Precision NanoSystems Inc. (PNI)
- Enzo Biochem Inc.
- Sirnaomics Inc.
- Arbutus Biopharma Corporation
- Biond Biologics
- Replicate Bioscience
- CureVac N.V.
- BioNTech SE
- Moderna Inc.
- Translate Bio (Sanofi)
- Alnylam Pharmaceuticals
- Roche
- Pfizer
- AstraZeneca
Frequently Asked Questions
What are Lipid Nanoparticles and their primary applications?
Lipid Nanoparticles (LNPs) are tiny lipid-based carriers designed to encapsulate and deliver therapeutic molecules like mRNA, DNA, and small drugs. Their primary applications include mRNA vaccines, gene therapy, and oncology treatments, offering improved drug stability and targeted delivery.
How is the Lipid Nanoparticles Market projected to grow?
The Lipid Nanoparticles Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2025 and 2032, driven by advancements in drug delivery systems and increasing demand for nucleic acid therapies.
What are the main drivers for the growth of the LNP market?
Key drivers include the success of mRNA vaccines, growing investments in gene therapy and personalized medicine research, technological advancements in LNP formulation, and the increasing prevalence of chronic diseases requiring targeted treatments.
Which regions are leading in the Lipid Nanoparticles Market?
North America and Europe are currently leading the Lipid Nanoparticles Market due to robust R&D infrastructure and significant investments. Asia Pacific is emerging as the fastest-growing region, propelled by increasing healthcare expenditure and biopharmaceutical manufacturing capabilities.
What role does AI play in the development of Lipid Nanoparticles?
AI plays a crucial role in optimizing LNP formulation design, predicting stability and in vivo performance, accelerating the discovery of new lipid components, and streamlining manufacturing processes, thereby enhancing efficiency and reducing development timelines.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
