Lyophilization Equipment Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427301 | Date : Oct, 2025 | Pages : 239 | Region : Global | Publisher : MRU
Lyophilization Equipment Market Size
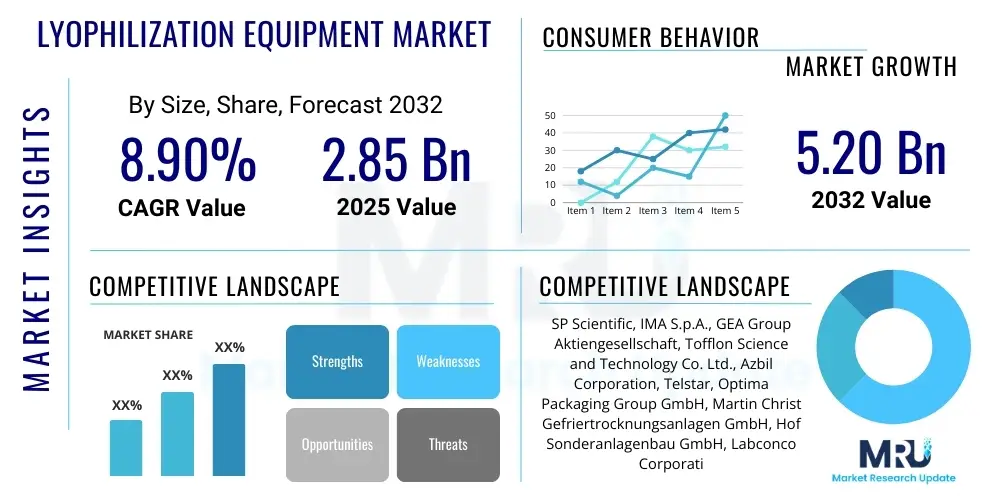
The Lyophilization Equipment Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.9% between 2025 and 2032. The market is estimated at USD 2.85 billion in 2025 and is projected to reach USD 5.20 billion by the end of the forecast period in 2032. This growth is primarily fueled by the escalating demand for stable and long-shelf-life pharmaceutical and biotechnology products, alongside increasing applications in the food processing and diagnostic sectors. Technological advancements leading to more efficient and automated systems also contribute significantly to this upward trajectory, addressing the evolving needs of various industries for high-quality product preservation.
Lyophilization Equipment Market introduction
The Lyophilization Equipment Market encompasses a specialized range of machinery designed for the freeze-drying process, which is critical for preserving the stability and extending the shelf-life of sensitive biological and chemical materials. This intricate process involves freezing the product, then reducing the surrounding pressure to allow the frozen water to sublimate directly from the solid phase to the gas phase, thereby removing moisture without affecting the products structure. Key products within this market include laboratory-scale, pilot-scale, and industrial-scale freeze dryers, differentiated by capacity, automation levels, and specific application requirements. Major applications span across pharmaceuticals and biotechnology for vaccines, biologics, and active pharmaceutical ingredients, as well as in the food industry for preserving high-value ingredients, and in the medical and diagnostic sectors for reagents and specimens. The primary benefits of lyophilization include superior product stability, reduced degradation, lower transportation costs due to decreased weight, and the ability to maintain the inherent biological activity or structural integrity of delicate substances. Significant driving factors include the rapid expansion of the biopharmaceutical sector, increasing global demand for stable and effective drug formulations, and continuous advancements in freeze-drying technology aimed at enhancing efficiency and reducing operational complexities.
Lyophilization Equipment Market Executive Summary
The Lyophilization Equipment Market is experiencing robust growth, driven by pivotal business trends such as the increasing automation in pharmaceutical manufacturing and the adoption of continuous lyophilization processes to enhance efficiency and throughput. Companies are heavily investing in advanced control systems and data analytics to optimize cycle times and ensure product quality, addressing the growing need for scalable and compliant solutions. From a regional perspective, North America and Europe continue to dominate the market due to significant research and development investments and the presence of major pharmaceutical and biotechnology companies, while the Asia-Pacific region is emerging as a high-growth market, propelled by expanding healthcare infrastructure, increasing biopharmaceutical production capabilities, and growing contract manufacturing activities. Segment-wise, the pharmaceutical and biotechnology applications segment retains the largest market share, driven by the continuous development of new biologics, vaccines, and injectable drugs that require sophisticated preservation methods. Concurrently, the food processing segment is witnessing a steady uptake of lyophilization equipment for premium food products and ingredients, reflecting a broader consumer demand for natural and minimally processed foods with extended shelf life.
AI Impact Analysis on Lyophilization Equipment Market
User inquiries concerning the impact of Artificial Intelligence on the Lyophilization Equipment Market frequently revolve around optimizing process parameters, improving predictive maintenance, and enhancing overall product quality and consistency. Common questions explore how AI can shorten lyophilization cycles, reduce energy consumption, and minimize human error, thereby leading to more cost-effective and efficient operations. There is also significant interest in AIs role in real-time monitoring and data analysis, which could provide deeper insights into the complex freeze-drying process and facilitate the development of novel formulations. Users are keen to understand how AI-driven analytics can identify critical process deviations before they impact product integrity, and how machine learning algorithms can learn from historical data to fine-tune future lyophilization protocols, making the entire process more robust and predictable. Another emerging theme is the potential for AI to support remote operation and troubleshooting, contributing to greater operational flexibility and reduced downtime for lyophilization facilities globally.
The integration of AI technologies promises to revolutionize various aspects of the lyophilization equipment lifecycle, from initial process development to ongoing operational management. By leveraging machine learning models, manufacturers and end-users can analyze vast datasets generated during freeze-drying cycles to identify optimal parameters for different product formulations, leading to faster development times and improved process scalability. This analytical capability moves beyond traditional empirical methods, offering a more scientific and data-driven approach to complex thermal and mass transfer challenges inherent in lyophilization. Furthermore, AI can contribute significantly to predictive maintenance strategies by monitoring equipment performance in real-time, detecting subtle anomalies that indicate potential failures, and scheduling maintenance proactively, thereby minimizing unexpected downtime and extending the lifespan of valuable assets. This proactive approach not only enhances operational efficiency but also safeguards product quality by ensuring equipment functions within precise specifications consistently.
- Enhanced Process Optimization: AI algorithms analyze data from numerous lyophilization cycles to identify optimal temperature, pressure, and time parameters, significantly reducing cycle duration and energy consumption.
- Predictive Maintenance: Machine learning models forecast potential equipment failures based on real-time sensor data, enabling proactive maintenance and minimizing unscheduled downtime.
- Improved Quality Assurance: AI systems monitor critical process parameters continuously, detecting deviations instantly and ensuring product consistency and quality throughout the batch.
- Accelerated R&D and Formulation Development: AI can simulate and predict the behavior of new formulations during freeze-drying, streamlining experimental design and speeding up the development of stable products.
- Aseptic Process Control: AI contributes to monitoring and maintaining aseptic conditions, crucial for pharmaceutical products, by detecting environmental anomalies and operational risks.
- Energy Efficiency: AI-driven control systems dynamically adjust energy usage based on real-time process needs, leading to significant reductions in operational costs and environmental impact.
DRO & Impact Forces Of Lyophilization Equipment Market
The Lyophilization Equipment Market is propelled by several key drivers, primarily the burgeoning biopharmaceutical industry and the increasing demand for stable, long-shelf-life drugs, vaccines, and biologics that often require freeze-drying for preservation. Technological advancements in equipment design, such as automation, continuous processing, and enhanced control systems, also significantly contribute to market growth by offering more efficient and reliable solutions. Opportunities abound in emerging markets, particularly in Asia-Pacific and Latin America, where healthcare infrastructure expansion and a rising focus on pharmaceutical manufacturing present fertile ground for market penetration. The adoption of lyophilization in new applications, such as advanced food processing for high-value ingredients and personalized medicine, further expands the markets potential. However, the market faces notable restraints, including the high capital investment required for purchasing and installing lyophilization equipment, coupled with the substantial operational costs associated with energy consumption and maintenance. The technical complexity of the lyophilization process itself, demanding specialized expertise and stringent regulatory compliance, also poses a significant barrier, particularly for smaller enterprises.
The impact forces influencing the Lyophilization Equipment Market are multifaceted, shaping its competitive landscape and strategic direction. The bargaining power of buyers, primarily large pharmaceutical and biotechnology companies, is moderate to high, as they often require customized, high-capacity solutions and can exert influence over pricing and features due to their purchasing volume. Conversely, the bargaining power of suppliers for critical components like vacuum pumps, refrigeration units, and control systems is also moderate, given the specialized nature and limited number of high-quality providers. The threat of new entrants is relatively low due to the high capital intensity, significant R&D investment, and the necessity for deep technical expertise and regulatory compliance in manufacturing sophisticated equipment. However, the threat of substitute products or processes, such as spray drying or other stabilization methods, exists, although for many biologicals and heat-sensitive compounds, lyophilization remains the gold standard, mitigating this threat to some extent. Intense competitive rivalry among existing players, characterized by continuous innovation, product differentiation, and strategic partnerships, is a defining feature of this market, driving advancements and market share battles.
Segmentation Analysis
The Lyophilization Equipment Market is comprehensively segmented across various dimensions to reflect its diverse applications, technological advancements, and end-user requirements. These segmentations provide a granular view of market dynamics, allowing for a deeper understanding of specific growth drivers and competitive landscapes within each category. The market is primarily divided by scale, addressing the different capacity needs from research and development to full-scale commercial production. Further distinctions are made by the type of equipment, reflecting different functional designs and operational principles. Applications and end-users form critical segmentation criteria, highlighting the primary industries and organizations that leverage this technology for product preservation and stabilization. This multi-faceted segmentation helps to identify key market niches, assess growth potential, and understand the strategic focus of market participants in delivering tailored solutions.
- By Scale:
- Laboratory Scale Lyophilizers: Primarily used for research, development, and small-batch production.
- Pilot Scale Lyophilizers: Bridge the gap between laboratory and commercial production, used for process optimization and clinical trial material manufacturing.
- Commercial Scale Lyophilizers: Large-capacity systems designed for mass production in pharmaceutical and food industries.
- By Type:
- Tray Freeze Dryers: Most common type, where products are placed on trays within a chamber.
- Manifold Freeze Dryers: Suitable for small volumes, where flasks are attached to a manifold.
- Rotary Freeze Dryers: Used for drying bulk materials in a rotating drum under vacuum.
- Benchtop Lyophilizers: Compact units for laboratory use.
- Industrial Lyophilizers: Large-scale, highly automated systems for high-volume manufacturing.
- By Application:
- Pharmaceutical & Biotechnology: Vaccines, biologics, injectables, APIs, diagnostics, blood plasma.
- Food Processing: Instant coffee, fruits, vegetables, dairy products, culinary ingredients.
- Medical & Healthcare: Surgical implants, tissue preservation, medical devices.
- Others: Cosmetics, cultural heritage preservation, chemical industries.
- By End-User:
- Pharmaceutical & Biotechnology Companies: Major consumers for drug and vaccine production.
- Contract Manufacturing Organizations (CMOs) & Contract Development and Manufacturing Organizations (CDMOs): Offer lyophilization services to other companies.
- Academic & Research Institutes: Utilize equipment for scientific studies and experimental development.
- Hospitals & Blood Banks: For preserving sensitive medical materials.
- Food & Beverage Companies: For premium food product preservation.
Lyophilization Equipment Market Value Chain Analysis
The value chain for the Lyophilization Equipment Market initiates with a robust upstream segment, comprising suppliers of highly specialized raw materials and precision components essential for equipment manufacturing. This includes providers of advanced vacuum pumps, sophisticated refrigeration systems, high-quality stainless steel for chambers, and intricate control systems and sensors crucial for precise process monitoring. These upstream partners play a vital role in ensuring the quality, reliability, and performance of the final lyophilization equipment. Manufacturers then assemble these components, integrating complex engineering with cutting-edge technology to produce various types of freeze dryers, ranging from laboratory benchtop units to large-scale industrial systems. The manufacturing stage also involves extensive research and development to innovate and improve equipment efficiency, automation capabilities, and compliance with stringent regulatory standards, particularly for pharmaceutical applications. Quality control and rigorous testing are paramount at this stage to ensure the equipment meets specified performance criteria and safety standards before reaching the market.
Moving downstream, the distribution channel for lyophilization equipment is multi-faceted, encompassing both direct sales and indirect channels to reach a diverse global customer base. For large-scale industrial units and customized solutions, direct sales are common, where equipment manufacturers engage directly with major pharmaceutical companies, biotechnology firms, and large-scale food processors. This direct approach often involves extensive consultations, technical support, installation services, and post-sales maintenance, fostering strong client relationships. Conversely, indirect distribution channels, primarily through a network of authorized distributors, agents, and value-added resellers, are critical for reaching smaller enterprises, academic institutions, and regional markets. These indirect partners often provide localized sales, technical support, and after-sales services, which are particularly important in regions where the manufacturer does not have a direct presence. The choice of distribution strategy often depends on the customers size, geographic location, and the complexity of the equipment being purchased, aiming to optimize market reach and customer service efficiency.
Effective management of both direct and indirect distribution channels is paramount for market penetration and sustaining competitive advantage. Direct channels allow for greater control over customer interactions, brand messaging, and pricing strategies, enabling manufacturers to build tailored solutions and provide specialized training. This approach is highly effective for high-value, complex equipment requiring significant technical expertise during sales and installation. Indirect channels, on the other hand, offer scalability and access to broader markets without the overhead of establishing extensive direct sales infrastructure. Distributors typically possess local market knowledge, established customer networks, and the ability to provide immediate support, which is invaluable for reaching a wider array of end-users, including small to medium-sized enterprises and research laboratories. Optimizing this dual-channel strategy, leveraging the strengths of both direct and indirect sales, is crucial for maximizing market share and ensuring comprehensive customer service across the entire product lifecycle.
Lyophilization Equipment Market Potential Customers
The primary potential customers for lyophilization equipment are diverse and span across industries that require the sophisticated preservation of sensitive and high-value products. Leading this group are pharmaceutical and biotechnology companies, which rely heavily on freeze-drying technology for the stabilization of a vast array of products including vaccines, biologics, monoclonal antibodies, active pharmaceutical ingredients (APIs), and various injectable drugs. The inherent instability of many modern therapeutics necessitates lyophilization to extend their shelf-life, maintain efficacy, and simplify storage and transportation. These companies often invest in both laboratory-scale units for research and development and large-scale commercial equipment for mass production, making them the largest end-user segment. Their continuous pipeline of new drug discoveries and the increasing complexity of biological formulations consistently drive the demand for advanced and efficient lyophilization solutions. The imperative for regulatory compliance and robust quality control further solidifies their position as key consumers, demanding equipment that offers precision, reliability, and scalability.
Another significant customer segment comprises Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs). These organizations offer specialized services, including lyophilization, to pharmaceutical and biotechnology firms that may lack in-house capabilities or require additional capacity. As the biopharmaceutical industry increasingly outsources various stages of drug development and manufacturing, the demand for CMOs/CDMOs equipped with state-of-the-art lyophilization equipment is steadily growing. Academic and research institutes also represent a substantial customer base, utilizing laboratory and pilot-scale lyophilizers for fundamental scientific research, experimental drug formulation, and the preservation of biological samples such such as DNA, RNA, and proteins. Their ongoing pursuit of scientific breakthroughs and the development of novel compounds ensures a consistent need for precise and versatile freeze-drying technology. Moreover, clinical laboratories and blood banks employ lyophilization for preserving diagnostic reagents, blood plasma, and other biological specimens, ensuring their stability and integrity over extended periods, which is crucial for accurate medical testing and patient care.
Beyond the core pharmaceutical and biotechnology sectors, the food and beverage industry constitutes a growing segment of potential customers. Manufacturers of premium food products, instant coffee, freeze-dried fruits, vegetables, and culinary ingredients utilize lyophilization to maintain the nutritional value, flavor, and texture of their products while significantly extending shelf-life without the need for refrigeration. This appeals to consumers seeking convenience and high-quality, minimally processed options. Furthermore, the medical device sector uses lyophilization for preserving certain implants and sterile components, while various other industries, including cosmetics for active ingredient preservation and cultural heritage institutions for artifact conservation, also represent niche but important customer bases for specialized lyophilization equipment. The common thread among all these potential customers is the critical need for effective moisture removal and preservation technology that upholds product quality and extends viability, underpinning the broad utility and growing market for lyophilization equipment.
Lyophilization Equipment Market Key Technology Landscape
The Lyophilization Equipment Market is characterized by a dynamic technology landscape, driven by continuous innovation aimed at enhancing efficiency, reducing processing times, and ensuring product integrity. A pivotal technological advancement is the integration of advanced control systems and automation, which allows for precise regulation of temperature, pressure, and time across various stages of the freeze-drying cycle. These systems often incorporate sophisticated sensors and programmable logic controllers (PLCs) to monitor critical process parameters in real-time, enabling automated adjustments and minimizing human intervention. This automation not only improves the reproducibility and consistency of the lyophilization process but also significantly reduces the risk of human error, which is paramount in the highly regulated pharmaceutical sector. The move towards fully automated loading and unloading systems, particularly in commercial-scale units, further optimizes throughput and minimizes aseptic risks by reducing manual contact with sterile products. This emphasis on automation directly addresses industry demands for increased operational efficiency and robust quality control, especially for high-volume production of sensitive biologicals.
Another critical area of technological development is Process Analytical Technology (PAT), which is gaining traction within the lyophilization domain. PAT involves the application of in-line or at-line analytical tools to monitor and control manufacturing processes in real-time, providing deep insights into product attributes and process performance. For lyophilization, PAT tools such as tunable diode laser absorption spectroscopy (TDLAS) for measuring water vapor, manometric temperature measurement (MTM) for determining product temperature, and capacitance manometers for vacuum pressure monitoring, are becoming increasingly common. These technologies enable a more scientific and data-driven approach to cycle development and optimization, moving away from traditional empirical methods. Furthermore, the development of continuous lyophilization systems represents a significant shift from traditional batch processing. Continuous systems offer the potential for improved scalability, reduced footprint, higher energy efficiency, and more consistent product quality due to tighter process control, aligning with the industrys push towards advanced manufacturing paradigms and industry 4.0 principles. While still evolving, continuous lyophilization promises to transform high-volume production of freeze-dried products by offering a streamlined and integrated approach.
Innovations in vacuum technology and refrigeration systems also form a crucial part of the technological landscape, contributing directly to the performance and energy efficiency of lyophilization equipment. Advanced vacuum pumps offer deeper vacuum levels and faster pump-down times, which are essential for efficient sublimation and reduced cycle durations. Simultaneously, more energy-efficient and environmentally friendly refrigeration systems are being developed, utilizing natural refrigerants and improved compressor technologies to lower operational costs and reduce environmental impact. Furthermore, there there is a growing focus on aseptic processing capabilities and sterile design, particularly for pharmaceutical applications, involving clean-in-place (CIP) and sterilize-in-place (SIP) functionalities to ensure stringent sterility requirements. The integration of smart sensors, data analytics platforms, and Internet of Things (IoT) connectivity also empowers predictive maintenance, remote monitoring, and data-driven decision-making, allowing manufacturers and end-users to optimize equipment performance, anticipate maintenance needs, and enhance overall operational reliability. These advancements collectively underscore a market moving towards more intelligent, efficient, and integrated lyophilization solutions.
Regional Highlights
- North America: Dominates the Lyophilization Equipment Market due to its robust pharmaceutical and biotechnology industries, significant R&D investments, and the presence of numerous key market players. The region benefits from strong government support for healthcare innovation and a high adoption rate of advanced manufacturing technologies.
- Europe: Represents a mature and substantial market, driven by established pharmaceutical companies, stringent regulatory standards, and a focus on advanced drug development. Countries like Germany, France, and the UK are key contributors, emphasizing technological advancements and high-quality equipment.
- Asia-Pacific: Emerging as the fastest-growing market, propelled by expanding healthcare infrastructure, increasing biopharmaceutical manufacturing activities, and rising contract manufacturing services. Countries such as China, India, and Japan are witnessing substantial investments in pharmaceutical production and research.
- Latin America: Showing steady growth, primarily due to improving healthcare access, increasing government expenditure on health, and the expansion of local pharmaceutical production capabilities. Brazil and Mexico are key markets within this region.
- Middle East & Africa: Exhibiting nascent growth, driven by increasing investments in healthcare infrastructure and a growing demand for stable pharmaceutical products. However, market development is slower compared to other regions due to economic and political instability in some areas.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Lyophilization Equipment Market.- SP Scientific
- IMA S.p.A.
- GEA Group Aktiengesellschaft
- Tofflon Science and Technology Co. Ltd.
- Azbil Corporation
- Telstar (a part of IDEX Corporation)
- Optima Packaging Group GmbH
- Martin Christ Gefriertrocknungsanlagen GmbH
- Hof Sonderanlagenbau GmbH
- Labconco Corporation
Frequently Asked Questions
What is lyophilization equipment and why is it crucial for pharmaceuticals?
Lyophilization equipment, also known as freeze dryers, removes water from frozen products via sublimation, preserving sensitive materials. It is crucial in pharmaceuticals for extending the shelf-life and maintaining the stability and biological activity of heat-sensitive drugs, vaccines, and biologics, ensuring their efficacy and safety over time.
What are the primary applications of lyophilization equipment beyond pharmaceuticals?
Beyond pharmaceuticals, lyophilization equipment is extensively used in the food industry for preserving high-value ingredients, instant coffee, and specialty foods, retaining their flavor, nutrition, and texture. It also finds applications in medical diagnostics for reagents, in biotechnology for enzyme and cell preservation, and in chemical industries.
How is AI impacting the efficiency and quality of lyophilization processes?
AI is transforming lyophilization by optimizing process parameters to reduce cycle times and energy consumption, enhancing predictive maintenance for equipment, and improving real-time quality assurance. It helps in developing new formulations faster and ensuring consistent product quality through data-driven insights.
What are the main factors driving the growth of the Lyophilization Equipment Market?
The markets growth is primarily driven by the expanding biopharmaceutical industry, increasing demand for stable and long-shelf-life drug formulations, and continuous technological advancements in equipment design, including automation and process analytical technology. Growth in food preservation and diagnostics also contributes significantly.
What are the key challenges faced by the lyophilization equipment market?
Key challenges include the high initial capital investment required for purchasing and installing advanced equipment, significant operational costs associated with energy consumption, and the technical complexities of the lyophilization process, which demands specialized expertise and strict regulatory compliance.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
