Non-animal Alternative Testing Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429916 | Date : Nov, 2025 | Pages : 241 | Region : Global | Publisher : MRU
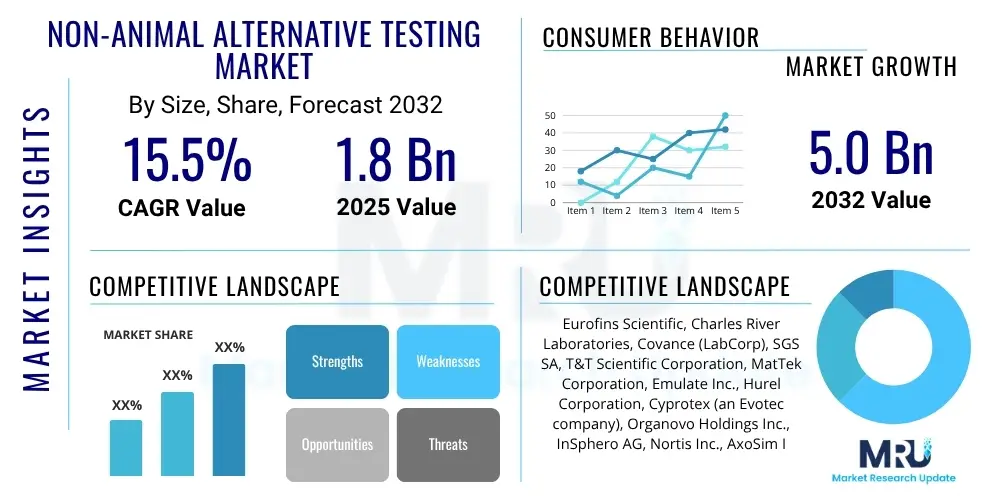
Market Value 2025: USD 1.8 Billion
Market Value 2032: USD 5.0 Billion
Non-animal Alternative Testing Market Size
The Non-animal Alternative Testing Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 15.5% between 2025 and 2032. The market is estimated at USD 1.8 Billion in 2025 and is projected to reach USD 5.0 Billion by the end of the forecast period in 2032.
Non-animal Alternative Testing Market introduction
The Non-animal Alternative Testing Market represents a transformative shift in scientific research and product development, moving away from conventional animal-based methodologies towards more ethical, human-relevant, and often more efficient approaches. This rapidly evolving sector encompasses a wide array of innovative technologies and techniques designed to evaluate the safety, efficacy, and toxicity of various substances without relying on live animals. The momentum for this transition is fueled by escalating ethical concerns regarding animal welfare, increasingly stringent global regulatory frameworks that encourage or mandate the reduction and replacement of animal testing, and the growing recognition of the inherent limitations of animal models in accurately predicting human biological responses due to significant species differences.
Product descriptions within this market include sophisticated in vitro models such as advanced 2D and 3D cell cultures, complex organoids and spheroids that mimic tissue structures, and human-on-a-chip systems that replicate organ-level physiology with remarkable fidelity. Furthermore, cutting-edge in silico methods, leveraging computational biology, machine learning, and artificial intelligence, play a pivotal role in predicting molecular interactions, biological pathways, and toxicological outcomes. These diverse technologies collectively aim to provide faster, more cost-effective, and highly predictive results for a broad spectrum of applications. The major applications span critical industries, including pharmaceutical and biopharmaceutical drug discovery and development, comprehensive toxicology screening for chemicals and industrial products, rigorous safety assessment in the global cosmetics industry, and essential food safety evaluation, demonstrating the widespread impact of these advancements.
The benefits associated with the adoption of non-animal alternative testing are extensive and multifaceted, extending beyond mere ethical considerations to encompass substantial scientific and economic advantages. These alternatives frequently yield more human-relevant data, which can significantly reduce the high failure rates of drug candidates in clinical trials that were previously deemed safe in traditional animal studies. They offer enhanced throughput capabilities, facilitating the screening of a larger volume of compounds in a considerably shorter timeframe, thereby accelerating innovation and product development cycles. Moreover, these methods can lead to substantial cost reductions by eliminating expenses associated with animal procurement, housing, specialized facilities, and lengthy experimental protocols. Key driving factors propelling this market forward include escalating public and consumer pressure for cruelty-free products and sustainable practices, evolving global regulations promoting the reduction, refinement, and replacement (3Rs) of animal testing, and continuous technological breakthroughs in fields such as stem cell research, microfluidics, and bioinformatics that enable the creation of increasingly complex, accurate, and human-relevant models. This convergence of ethical imperatives, scientific progress, and regulatory evolution underscores the immense potential and critical importance of the non-animal alternative testing market as a cornerstone of modern scientific endeavor.
Non-animal Alternative Testing Market Executive Summary
The Non-animal Alternative Testing Market is poised for substantial expansion, propelled by a strong interplay of ethical motivations, scientific advancements, and regulatory pressures. Current business trends indicate a significant surge in research and development investments directed towards pioneering novel in vitro and in silico methodologies. Pharmaceutical and biotechnology companies are increasingly integrating these sophisticated testing platforms into their early-stage drug discovery and preclinical development processes. This strategic shift aims to enhance predictive accuracy, mitigate attrition rates in clinical trials, and accelerate the identification of viable therapeutic candidates. Furthermore, a prominent trend involves the formation of strategic collaborations between technology developers, academic research institutions, and end-user industries. These partnerships are crucial for expediting the validation, standardization, and widespread adoption of innovative alternative methods, fostering a dynamic and competitive market landscape where both established corporations and agile startups are vying for market leadership through continuous technological innovation and the expansion of specialized service offerings, including contract research organizations focused exclusively on non-animal testing solutions.
From a regional perspective, North America and Europe currently represent the dominant forces within the market. This leadership is primarily attributed to robust regulatory frameworks actively supporting the reduction of animal testing, substantial governmental and private funding for advanced R&D, and a high degree of public awareness and advocacy concerning animal welfare. These regions are at the forefront of developing, validating, and implementing sophisticated non-animal testing strategies, particularly evident in the highly regulated cosmetics and chemical industries. However, the Asia Pacific region is rapidly emerging as a significant growth engine, driven by escalating investments in life sciences research, a burgeoning consumer demand for cruelty-free and ethically produced goods, and the progressive alignment with international testing guidelines. Countries such as China, India, and South Korea are experiencing a notable acceleration in research activities and infrastructure development, positioning them as pivotal contributors to market growth throughout the forecast period. Latin America, along with the Middle East and Africa, also exhibit nascent yet promising growth trajectories, stimulated by increasing awareness of ethical testing practices and the expansion of their respective healthcare and consumer goods sectors.
Segmentation trends within the market highlight that in vitro testing methods, particularly advanced cell culture techniques, presently command the largest market share due to their inherent versatility, increasing sophistication, and direct applicability to human cell biology. Nevertheless, the organ-on-a-chip segment is projected to achieve the highest compound annual growth rate, reflecting its immense potential to meticulously mimic human organ physiology and complex disease states with unprecedented accuracy. This capability offers highly relevant data for crucial applications in drug screening, toxicity assessment, and disease modeling. Concurrently, in silico methods are gaining significant traction, especially with advancements in artificial intelligence and machine learning, for their unparalleled ability to computationally predict toxicity and efficacy, thereby complementing and enhancing experimental approaches. The pharmaceutical and biopharmaceutical sector continues to be the largest end-user, primarily driven by the imperative for more efficient, predictive, and ethical drug development pathways. The cosmetics industry remains a crucial accelerator, particularly in jurisdictions with explicit prohibitions on animal testing for cosmetic products. Overall, the market is well-positioned for sustained and vigorous expansion, underpinned by continuous innovation, profound ethical considerations, and a fundamental global paradigm shift towards more human-relevant and scientifically robust inquiry.
AI Impact Analysis on Non-animal Alternative Testing Market
Users frequently inquire about the transformative impact of Artificial Intelligence (AI) on the Non-animal Alternative Testing Market, with a strong focus on its potential to significantly enhance predictive accuracy, accelerate the discovery of novel compounds, and fundamentally reduce the reliance on conventional animal models. Key thematic concerns and expectations revolve around AI's exceptional capability to meticulously analyze vast and complex datasets derived from in vitro and in silico experiments, identify intricate patterns that might elude human observation, and predict biological outcomes with substantially greater precision. There are considerable expectations regarding AI's pivotal role in areas such as drug repurposing, advancing personalized medicine, and facilitating the rapid, high-throughput screening of extensive chemical libraries for potential toxicity. However, alongside these promising outlooks, pertinent concerns also emerge regarding the robust validation of AI models, the critical issue of explainability in their predictions, the seamless integration of diverse data types from various sources, and the broader ethical implications associated with the increasing autonomy of AI in scientific decision-making. Users are particularly keen to understand how AI can effectively surmount the challenges inherent in modeling the complexity of biological systems and bridge the crucial gap between results obtained from in vitro experiments and their ultimate relevance in vivo.
- AI significantly accelerates drug discovery processes by rapidly screening extensive libraries of chemical compounds, predicting their potential efficacy and toxicity, thereby substantially reducing the need for prolonged and resource-intensive experimental animal testing phases.
- Advanced predictive toxicology models, powered by sophisticated machine learning algorithms, meticulously analyze molecular structures and vast repositories of existing toxicity data to accurately forecast adverse effects, leading to improved safety assessments for novel chemicals and pharmaceutical agents.
- AI plays a crucial role in the intelligent design and optimization of intricate in vitro models, such as sophisticated organ-on-a-chip systems and 3D bioprinted tissues, by simulating complex cellular interactions and precisely predicting optimal culture conditions for enhanced physiological relevance.
- The enhanced data analysis capabilities of AI empower researchers to extract profound and actionable insights from complex omics data (including genomics, proteomics, and metabolomics) generated from non-animal tests, offering a deeper and more comprehensive understanding of underlying biological mechanisms.
- AI facilitates the advancement of personalized medicine by meticulously identifying individual responses to specific drugs based on unique genetic and cellular profiles derived from patient-specific in vitro models, thereby moving beyond generalized animal responses to offer tailored therapeutic strategies.
- It significantly improves the developmental pipeline for novel alternative methods by identifying subtle patterns within experimental results and subsequently suggesting innovative approaches or promising targets for further investigation and validation.
- AI automates and streamlines complex image analysis and data interpretation in high-throughput screening assays, dramatically increasing both the efficiency and consistency of non-animal testing workflows, leading to more reliable and reproducible outcomes.
DRO & Impact Forces Of Non-animal Alternative Testing Market
The Non-animal Alternative Testing Market is profoundly shaped by a dynamic interplay of potent drivers, notable restraints, and significant opportunities, which collectively act as powerful impact forces determining its growth trajectory. A primary and pervasive driver is the escalating ethical opposition to animal testing, a sentiment that has garnered substantial public support globally and fueled robust advocacy efforts from numerous animal welfare organizations. This strong ethical imperative translates directly into heightened consumer demand for cruelty-free products, particularly within the influential cosmetics and personal care sectors, thereby compelling industries to actively seek, develop, and adopt alternative testing methodologies. Concurrently, the evolving and increasingly stringent regulatory landscapes, notably exemplified by directives in the European Union, India, and Israel, are mandating or strongly encouraging the reduction, refinement, and replacement (3Rs) of animal testing, thereby establishing a critical legal and commercial impetus for sustained market growth. Furthermore, continuous technological advancements, including revolutionary breakthroughs in stem cell technology, sophisticated microfluidics, and advanced computational biology, are persistently expanding the capabilities and enhancing the scientific relevance of non-animal models, making them increasingly attractive and viable alternatives to traditional animal-based methods.
Despite these compelling drivers, the market navigates several notable restraints that pose challenges to its rapid and universal adoption. One significant hurdle is the considerable initial cost associated with the research, development, rigorous validation, and subsequent implementation of new alternative testing methods. Establishing sophisticated in vitro platforms, developing complex and robust in silico models, and training specialized scientific personnel to operate these advanced systems necessitate substantial capital investment, which can be prohibitive for smaller entities. Moreover, the enduring lack of universal standardization and widespread regulatory acceptance across all industrial sectors and diverse geographical regions remains a formidable impediment. While some alternative methods have undergone rigorous validation and gained approval, comprehensive regulatory endorsement for all applications is still an ongoing process, leading to a somewhat fragmented adoption landscape. Furthermore, a degree of skepticism from a segment of the scientific community concerning the full predictive capacity and ability of alternative models to accurately replicate the systemic complexity of an entire living organism, particularly for systemic effects, also acts as a restraint, underscoring the ongoing need for further validation studies, robust data, and targeted educational initiatives to build widespread scientific confidence.
However, these challenges are powerfully counterbalanced by significant opportunities that are ripe for market expansion and innovative breakthroughs. The burgeoning demand for personalized medicine presents an exceptionally fertile ground for non-animal models, as these can be meticulously tailored to individual patient profiles, thereby offering more precise drug development strategies and highly targeted therapeutic interventions. The vast and inherently complex field of drug discovery and development consistently seeks faster, more accurate, and ultimately more cost-effective methods, positioning non-animal alternative testing as a crucial solution to mitigate the notoriously high attrition rates of drug candidates during preclinical and clinical phases. Furthermore, the increasing complexity of environmental toxicology and chemical safety assessment, driven by the continuous development of new substances and evolving regulatory mandates, generates a persistent and growing need for advanced, high-throughput, and human-relevant testing platforms. The immense potential for synergistic collaborations between industry stakeholders, academic researchers, and regulatory bodies to pool resources, share invaluable knowledge, and collectively expedite the validation process represents a powerful opportunity to overcome current restraints, accelerate adoption, and unlock the full transformative potential of this critical and dynamic market.
Segmentation Analysis
The Non-animal Alternative Testing Market is meticulously segmented to provide a granular and comprehensive understanding of its diverse components, encompassing various technologies, applications, and end-users. This detailed segmentation is crucial for developing targeted market strategies, identifying specific high-growth areas, and comprehending the intricate dynamics within the broader market landscape. The technologies employed in this market are diverse and increasingly sophisticated, with each offering distinct advantages tailored to specific testing requirements and scientific inquiries. Applications span a wide range of critical sectors, from healthcare and pharmaceuticals to consumer goods and environmental safety, reflecting the inherent versatility and broad applicability of non-animal testing methods across multiple industries. The end-user analysis further refines this understanding by identifying the key industries, institutions, and organizations that are primarily driving the demand for and adoption of these innovative solutions, thereby underscoring where the most significant investments, innovations, and shifts in competitive dynamics are currently occurring, which will ultimately shape the market's future trajectory.
- By Technology:
- In Vitro Testing
- 2D Cell Culture: Traditional monolayer cell cultures, foundational for basic cell biology studies.
- 3D Cell Culture: More advanced models including organoids and spheroids, mimicking physiological architecture and function.
- Co-Culture Systems: Models involving interaction between different cell types or tissues.
- Microfluidics (Organ-on-a-Chip): Advanced systems that simulate organ functions and interactions.
- High-Throughput Screening (HTS): Automated methods for rapidly testing large numbers of compounds.
- In Silico Testing
- Quantitative Structure-Activity Relationships (QSAR): Computational models predicting biological activity from chemical structure.
- Molecular Modeling: Simulations of molecular interactions and dynamics.
- Machine Learning & Artificial Intelligence: Algorithms for predictive analysis, data pattern recognition, and model optimization.
- Systems Biology: Holistic computational modeling of biological systems.
- Ex Vivo Testing (Human Tissues, Biopsies): Utilizing freshly excised human tissues or biopsies for testing.
- Other Technologies: Including toxicogenomics, proteomics, and advanced imaging techniques.
- In Vitro Testing
- By Application:
- Drug Discovery and Development
- Drug Efficacy Testing: Assessing the effectiveness of potential therapeutic compounds.
- Drug Toxicity Screening: Identifying adverse effects of drug candidates.
- Pharmacokinetics & Pharmacodynamics: Studying drug absorption, distribution, metabolism, excretion, and mechanism of action.
- Toxicology Testing
- Dermal Toxicity: Assessing skin irritation and sensitization.
- Ocular Toxicity: Evaluating potential eye damage.
- Genotoxicity: Detecting DNA damage.
- Cytotoxicity: Measuring cell viability and health.
- Neurotoxicity: Investigating effects on the nervous system.
- Reproductive and Developmental Toxicity: Assessing impacts on reproduction and embryonic development.
- Cosmetics & Personal Care Product Testing: Safety assessment for ingredients and finished cosmetic products.
- Chemical Testing: Evaluating industrial chemicals for environmental and human safety.
- Food & Beverage Testing: Ensuring the safety and quality of food ingredients and products.
- Medical Device Testing: Assessing biocompatibility and safety of medical devices.
- Disease Modeling: Creating in vitro models to study disease mechanisms and test therapies.
- Drug Discovery and Development
- By End User:
- Pharmaceutical & Biopharmaceutical Companies: Major consumers for drug development and safety assessment.
- Cosmetics & Personal Care Industry: Driven by ethical concerns and regulatory mandates for cruelty-free products.
- Chemical Industry: Utilizes alternatives for chemical safety and regulatory compliance.
- Food & Beverage Industry: For product safety, quality control, and ingredient assessment.
- Academic & Research Institutes: For fundamental research, disease modeling, and new method development.
- Contract Research Organizations (CROs): Specialized service providers offering non-animal testing.
- Government Agencies & Regulatory Bodies: Influencers and funders of alternative method development and validation.
Value Chain Analysis For Non-animal Alternative Testing Market
The value chain for the Non-animal Alternative Testing Market is a dynamic and intricately linked ecosystem, commencing with fundamental upstream research and development activities and extending seamlessly through to the diverse downstream end-users. Upstream analysis critically highlights the pivotal role of foundational scientific research in burgeoning fields such as stem cell biology, sophisticated tissue engineering, advanced bioinformatics, and microfluidics. These areas form the indispensable bedrock for conceiving and developing novel alternative models and testing methodologies. Key players in this segment include suppliers of essential raw materials and highly specialized components, which encompass a vast array of high-quality cell lines, primary cells, specialized culture media, a diverse range of reagents, specific antibodies, precision microfluidic devices, and cutting-edge laboratory equipment. Academic institutions and innovative biotech startups frequently drive the initial phases of innovation, providing crucial foundational technologies and intellectual property that are subsequently prepared for commercialization. This initial segment is primarily focused on the creation of the fundamental tools, platforms, and methodologies essential for robust and reliable alternative testing.
The midstream segment of the value chain is characterized by the intensive activities of development, manufacturing, and commercialization of specific non-animal testing products and a comprehensive suite of services. This includes a growing number of specialized companies that produce highly sophisticated in vitro models, such as advanced organ-on-a-chip platforms, state-of-the-art 3D bioprinters, and integrated high-throughput screening systems. This segment also encompasses a burgeoning network of contract research organizations (CROs) that specialize in delivering bespoke non-animal testing services to industries that may lack the requisite in-house capabilities or prefer to seek external, validated expertise. Furthermore, software developers contributing to sophisticated in silico modeling platforms, intricate AI algorithms for advanced data analysis, and comprehensive bioinformatics tools constitute a critical and rapidly expanding component of this stage. Paramount importance is placed on stringent quality control, rigorous validation processes, and meticulous adherence to regulatory compliance throughout this stage to ensure the reliability, reproducibility, and ultimate widespread acceptance of alternative methods by both scientific and regulatory bodies.
Downstream analysis focuses intently on the diverse distribution channels and the ultimate end-users of non-animal alternative testing solutions. Distribution channels within this market are varied and strategically deployed, encompassing direct sales from manufacturers to large-scale pharmaceutical companies, major academic institutions, or government agencies. They also include indirect channels facilitated by specialized distributors who possess the expertise and network to cater to a broader and more diverse range of clients, including smaller biotech firms and research laboratories. Online platforms, industry-specific marketplaces, and influential scientific conferences also serve as increasingly important avenues for market reach, product dissemination, and knowledge exchange. The end-users are remarkably diverse, comprising pharmaceutical and biopharmaceutical companies striving to accelerate drug discovery, reduce animal usage, and improve human relevance; cosmetics and personal care manufacturers driven by ethical consumer demand and strict regulatory bans; chemical and food industries requiring comprehensive product safety assessments; and academic and research institutions engaged in fundamental and translational research. Both direct sales strategies targeting large corporations and indirect sales through distributors to smaller laboratories and emerging companies characterize the market’s go-to-market approaches, with a persistent and growing emphasis on developing tailored solutions for the specific and evolving needs of each client segment.
Non-animal Alternative Testing Market Potential Customers
The Non-animal Alternative Testing Market serves an extensive array of end-users and buyers, all united by a common objective to adopt more ethical, efficient, and human-relevant methods for critical processes such as product development, safety assessment, and foundational scientific research. Pharmaceutical and biopharmaceutical companies constitute a foundational and profoundly significant customer segment. These entities operate under immense pressure to mitigate the alarmingly high attrition rates of drug candidates during clinical trials, a pervasive challenge often attributed to the inadequate predictive power of traditional animal models. Consequently, they are increasingly leveraging non-animal alternatives for early-stage drug discovery, comprehensive efficacy testing, rigorous toxicity screening, and sophisticated personalized medicine approaches. Their primary goal is to identify promising compounds more rapidly and with greater confidence in their human relevance, thereby streamlining their research and development processes, significantly reducing substantial costs associated with animal husbandry, and aligning their operations with evolving global ethical standards and regulatory expectations.
Another considerably substantial customer base resides within the dynamic cosmetics and personal care industry. This sector is powerfully driven by strict regulatory prohibitions on animal testing for cosmetic products in major markets such as the European Union, India, Israel, and other regions, coupled with overwhelming consumer demand for cruelty-free and ethically produced goods globally. Consequently, this industry stands as a leading and proactive adopter of non-animal alternative testing methodologies. Companies in this domain extensively employ sophisticated in vitro models for the comprehensive safety assessment of both raw ingredients and finished products, encompassing a wide range of tests for potential skin irritation, sensitization, and phototoxicity. Similarly, the chemical industry forms a robust customer segment, facing stringent regulations like REACH in Europe that necessitate exhaustive safety assessments for thousands of chemical substances. Non-animal methods offer efficient, cost-effective, and rapid means to screen for various toxicological endpoints, including genotoxicity, carcinogenicity, and developmental toxicity, thereby facilitating compliance with regulatory mandates and supporting broader sustainability objectives.
Furthermore, academic and research institutes represent vital potential customers, actively utilizing non-animal models for conducting fundamental biological research, intricate disease modeling, and the critical development and validation of novel testing methodologies. These institutions are instrumental in driving scientific innovation and providing essential pathways for the independent validation and acceptance of emerging alternative technologies. Contract Research Organizations (CROs) that specialize in non-animal testing services are also key buyers, providing invaluable outsourced expertise to companies that either lack the internal infrastructure, possess limited specialized knowledge, or require independent validation of their own findings. While not direct buyers of products in the traditional sense, government agencies and regulatory bodies are critical stakeholders, profoundly influencing market demand by funding crucial research, developing robust guidelines, and ultimately accepting or mandating the use of these alternative methods. The sheer diversity and expanding needs of these potential customers underscore the widespread applicability, increasing necessity, and burgeoning growth trajectory of non-animal alternative testing solutions across a multitude of scientific, industrial, and societal domains.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 1.8 Billion |
| Market Forecast in 2032 | USD 5.0 Billion |
| Growth Rate | CAGR 15.5% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Eurofins Scientific, Charles River Laboratories, Covance (LabCorp), SGS SA, T&T Scientific Corporation, MatTek Corporation, Emulate Inc., Hurel Corporation, Cyprotex (an Evotec company), Organovo Holdings Inc., InSphero AG, Nortis Inc., AxoSim Inc., ALTEX (Alternatives to Animal Experimentation), BioIVT, Enzo Biochem Inc., Xenometrix AG, Promega Corporation, Thermo Fisher Scientific, Lonza Group. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Non-animal Alternative Testing Market Key Technology Landscape
The Non-animal Alternative Testing Market is fundamentally characterized by a rapidly evolving and highly innovative technological landscape, which consistently pushes the boundaries of scientific research and development. At the forefront of this transformation are advanced in vitro methodologies, encompassing a vast array of sophisticated cell culture techniques. These range from conventional 2D cell cultures, which provide foundational data for basic cellular biology studies, to increasingly complex and highly sophisticated 3D cell cultures. The 3D models include physiologically relevant spheroids and organoids, often derived from stem cells or primary human cells, which are capable of more accurately mimicking the architectural structure and intricate functions of native tissues and organs. These advanced models offer a superior platform for comprehensive drug screening, realistic disease modeling, and detailed toxicology studies compared to traditional monolayer cultures. Integral to this landscape are High-Throughput Screening (HTS) platforms, which enable the rapid and cost-effective testing of thousands of compounds simultaneously, frequently integrated with automated liquid handling and robotic systems to significantly accelerate research workflows and increase efficiency.
A particularly groundbreaking innovation driving the market is the development of microfluidics-based systems, widely recognized as organ-on-a-chip technology. These miniaturized devices meticulously incorporate living cells within precisely engineered micro-environments to recapitulate the complex physiological functions and mechanical stimuli of various human organs, such as the lung, liver, heart, brain, and kidney. Even more advanced multi-organ-on-a-chip systems are progressively emerging, specifically designed to model systemic interactions and cross-talk between different organs, providing an even greater degree of human relevance for intricate studies involving drug metabolism, systemic toxicity, and disease progression. Complementing these experimental techniques, in silico methods are playing an increasingly critical and indispensable role. These computational approaches primarily include Quantitative Structure-Activity Relationships (QSAR), which are employed to predict biological activity based on a chemical's molecular structure, and sophisticated molecular modeling techniques that simulate detailed drug-receptor interactions. The powerful integration of advanced machine learning and artificial intelligence algorithms is further significantly enhancing the predictive capabilities of in silico approaches, enabling the analysis of colossal datasets and the identification of complex, non-obvious patterns that profoundly inform drug design and comprehensive toxicity assessment.
Beyond these established and emerging technologies, innovative platforms such as 3D bioprinting are making significant strides, enabling the precise creation of complex, multi-cellular tissue constructs with exact spatial arrangements. This capability is paving the way for the development of even more realistic and functional in vitro models for applications ranging from regenerative medicine to advanced drug testing. Furthermore, cutting-edge omics technologies, including toxicogenomics and proteomics, which meticulously study changes in gene and protein expression in response to toxic substances, offer comprehensive and mechanistic insights into the molecular pathways of toxicity at an exceptionally early stage of investigation. These diverse technologies collectively contribute to a more predictive, highly efficient, and ethically sound approach to scientific testing. The continuous evolution, synergistic integration, and rapid convergence of these varied technological advancements are fundamental drivers of the market's robust growth, steering the transition away from animal models and towards more accurate, human-relevant, and ultimately safer scientific discovery and comprehensive product safety assessment, leading to the development of safer consumer products and more effective therapeutic interventions.
Regional Highlights
- North America: This region stands as a formidable and leading force in the Non-animal Alternative Testing Market, notably characterized by substantial and sustained investments in research and development, particularly emanating from the United States and Canada. The presence of robust government funding initiatives for life sciences, synergistically coupled with a strong concentration of major pharmaceutical, biotechnology, and chemical companies, actively drives innovation and accelerates the adoption of alternative testing methods. The region also benefits from a high level of public awareness and advocacy regarding animal welfare, which provides significant societal support for the regulatory impetus towards the widespread implementation of non-animal alternatives. Leading academic institutions and a thriving startup ecosystem contribute profoundly to the continuous development and commercialization of advanced in vitro and in silico models.
- Europe: Europe holds a pioneering position in the market, largely due to its proactive and stringent regulatory landscape, epitomized by the EU ban on animal testing for cosmetics and subsequent ambitious efforts to promote and mandate alternative testing methods for chemicals under regulations like REACH. This progressive regulatory environment has effectively fostered a culture of scientific innovation and widespread adoption of non-animal alternatives across a diverse array of industries. Key contributing countries such as Germany, the United Kingdom, France, and Switzerland boast robust research infrastructures, a significant number of specialized Contract Research Organizations (CROs), and substantial private and public funding dedicated to the development and validation of alternative methods. The European Centre for the Validation of Alternative Methods (ECVAM) plays a crucial and influential role in promoting standardization and achieving regulatory acceptance throughout the continent.
- Asia Pacific (APAC): The APAC region is rapidly emerging as a high-growth and strategically important market, primarily driven by increasing expenditures in research and development, particularly within economic powerhouses like China, Japan, South Korea, and India. While some countries within this diverse region have historically relied heavily on animal testing, there is a discernable and growing trend towards adopting international standards and ethical scientific practices. The rapidly expanding pharmaceutical, biotechnology, and cosmetics sectors, coupled with a burgeoning middle class and increasing consumer demand for diverse and ethically produced consumer products, are collectively stimulating a compelling need for efficient, predictive, and humane testing solutions. Significant investments in advanced scientific infrastructure and a growing pool of highly skilled researchers are further propelling the market forward, positioning APAC as a pivotal growth engine.
- Latin America: This region is currently experiencing a nascent but steadily accelerating adoption of non-animal alternative testing methodologies. Countries such as Brazil and Mexico are leading the charge, significantly influenced by global ethical trends and the gradual harmonization of their national regulatory frameworks with international standards that promote alternative methods. While the market in Latin America is still in its formative stages compared to the more established markets of North America and Europe, increasing foreign direct investment in the pharmaceutical and cosmetics sectors, alongside local initiatives championing animal welfare, are anticipated to fuel substantial market expansion throughout the forecast period, fostering a transition towards more ethical research practices.
- Middle East and Africa (MEA): The MEA market for non-animal alternative testing is also in a developing phase, characterized by incremental but consistent growth. This expansion is primarily driven by the region's expanding healthcare infrastructure, a heightened awareness of ethical scientific practices, and the growing presence and influence of international pharmaceutical and cosmetic companies within the region. Strategic investments in scientific research, coupled with targeted educational initiatives, are gradually paving the way for greater adoption of alternative testing solutions, particularly within economically developing countries like Saudi Arabia and South Africa. Anticipated advancements in regional regulatory frameworks and increased inter-regional collaborations are expected to significantly accelerate market penetration and growth in the coming years.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Non-animal Alternative Testing Market.- Eurofins Scientific
- Charles River Laboratories
- Covance (LabCorp)
- SGS SA
- T&T Scientific Corporation
- MatTek Corporation
- Emulate Inc.
- Hurel Corporation
- Cyprotex (an Evotec company)
- Organovo Holdings Inc.
- InSphero AG
- Nortis Inc.
- AxoSim Inc.
- ALTEX (Alternatives to Animal Experimentation)
- BioIVT
- Enzo Biochem Inc.
- Xenometrix AG
- Promega Corporation
- Thermo Fisher Scientific
- Lonza Group
Frequently Asked Questions
What is non-animal alternative testing?
Non-animal alternative testing encompasses a range of scientific methods and technologies, such as in vitro cell cultures, organ-on-a-chip systems, and in silico computational models, designed to assess product safety, efficacy, and toxicity without using live animals. These methods aim to provide more human-relevant data, reduce ethical concerns, and accelerate research and development processes.
Why is there a growing demand for non-animal alternative testing?
The demand is driven by several factors, including escalating ethical concerns regarding animal welfare, stringent global regulations that promote or mandate the reduction and replacement of animal testing, and the scientific limitations of animal models in accurately predicting human responses. Additionally, technological advancements offer more predictive and efficient alternatives.
What are the primary benefits of using non-animal testing methods?
The key benefits include improved ethical considerations by avoiding animal suffering, enhanced scientific relevance through human-specific models, faster turnaround times for screening, and potential cost reductions compared to traditional animal studies. These methods also facilitate high-throughput screening and personalized medicine approaches.
What are the main challenges faced by the non-animal alternative testing market?
Significant challenges include the high initial investment required for developing and validating new methods, the lack of universal standardization and regulatory acceptance across all applications and regions, and a degree of skepticism within the scientific community regarding the complexity and predictive power of some alternative models for systemic effects.
How does AI impact the non-animal alternative testing market?
AI significantly impacts the market by enhancing predictive toxicology, accelerating drug discovery through rapid compound screening, optimizing in vitro model design, and enabling advanced analysis of complex biological data. AI-powered tools improve efficiency, accuracy, and the overall predictive capacity of non-animal tests.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
