NUT Midline Carcinoma Treatment Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429885 | Date : Nov, 2025 | Pages : 248 | Region : Global | Publisher : MRU
NUT Midline Carcinoma Treatment Market Size
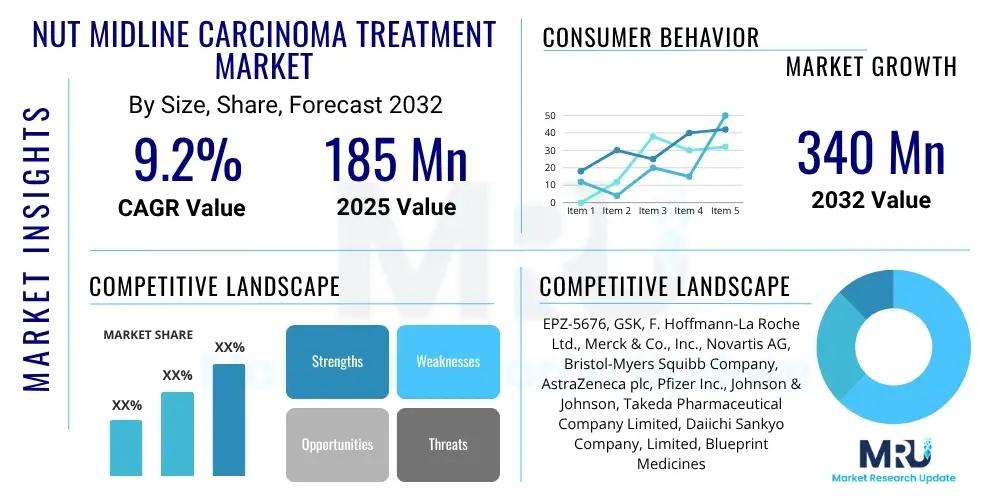
The NUT Midline Carcinoma Treatment Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.2% between 2025 and 2032. The market is estimated at USD 185 million in 2025 and is projected to reach USD 340 million by the end of the forecast period in 2032.
NUT Midline Carcinoma Treatment Market introduction
NUT Midline Carcinoma (NMC) represents an aggressive and rare form of cancer characterized by chromosomal rearrangements involving the NUT (Nuclear Protein in Testis) gene, most commonly t(15;19) (q13;p13.1) translocations. This distinct genetic signature leads to the formation of oncogenic fusion proteins, primarily BRD4-NUT or BRD3-NUT, which drive uncontrolled cell proliferation and inhibit differentiation. The disease can originate in various midline structures, including the head and neck, thorax, and abdominal cavities, often presenting with rapidly growing masses and a poor prognosis due to its aggressive nature and resistance to conventional therapies. Understanding the molecular underpinnings of NMC is crucial for developing targeted interventions, as its rarity poses significant challenges for diagnosis and treatment standardization.
The product description for the NUT Midline Carcinoma Treatment Market encompasses a range of therapeutic interventions aimed at combating this rare and challenging cancer. These treatments span from traditional approaches like chemotherapy and radiation therapy to innovative targeted therapies, immunotherapies, and epigenetic modulators. Given the specific genetic driver of NMC, Bromodomain and Extraterminal Domain (BET) inhibitors have emerged as a promising class of targeted drugs, specifically designed to disrupt the oncogenic activity of the NUT fusion proteins. Research and development efforts are continuously focused on identifying novel compounds and combination regimens that can effectively overcome therapeutic resistance and improve patient outcomes, including advanced biologics and cell-based therapies.
Major applications of NUT Midline Carcinoma treatments are predominantly found within specialized oncology centers, academic research institutions, and large hospital networks equipped with advanced diagnostic and therapeutic capabilities. These facilities cater to patients requiring complex multidisciplinary care, including precise molecular diagnostics for accurate identification of NUT fusions, personalized treatment planning, and access to clinical trials. The benefits of advancements in this market are profound, offering the potential for improved overall survival rates, enhanced quality of life through more targeted and less toxic treatments, and better disease control for patients who previously had limited options. Driving factors for market growth include the increasing understanding of NMC's molecular pathology, rising investment in oncology research, the growing adoption of precision medicine, and a strong pipeline of innovative drugs targeting specific oncogenic pathways.
NUT Midline Carcinoma Treatment Market Executive Summary
The NUT Midline Carcinoma Treatment Market is undergoing significant evolution, driven by a deeper understanding of its genetic basis and the emergence of targeted therapies. Business trends indicate a robust focus on strategic collaborations between pharmaceutical companies, biotechnology firms, and academic institutions to accelerate drug discovery and clinical development for this orphan disease. Companies are increasingly investing in advanced diagnostic tools, such as next-generation sequencing, to ensure early and accurate identification of NUT fusions, which is critical for guiding treatment decisions. Furthermore, the market is characterized by a strong emphasis on orphan drug designations and accelerated regulatory pathways, reflecting the high unmet medical need and encouraging R&D investment in this niche therapeutic area.
Regional trends reveal varying levels of market maturity and research activity. North America and Europe currently dominate the market due to well-established healthcare infrastructures, significant R&D spending, and a higher number of ongoing clinical trials for rare cancers. These regions benefit from supportive regulatory frameworks that facilitate the approval of novel therapies. However, the Asia Pacific region is anticipated to exhibit the fastest growth over the forecast period, primarily fueled by improving healthcare access, increasing awareness, and a rising prevalence of cancer cases, coupled with growing investments in precision oncology by local and international players. Latin America, the Middle East, and Africa are also showing nascent growth, driven by efforts to improve diagnostic capabilities and access to specialized treatments, albeit at a slower pace.
Segmentation trends highlight the increasing prominence of targeted therapies, particularly BET inhibitors, as the cornerstone of future NMC treatment paradigms, gradually shifting away from conventional chemotherapy as a primary standalone option. Drug development is heavily focused on novel small molecules and biologics that specifically interfere with the BRD4-NUT fusion protein's activity. From an end-user perspective, specialized oncology centers and research hospitals remain the primary consumers of these advanced treatments, emphasizing the highly specialized nature of NMC care. The market is also seeing a rise in combination therapies, exploring synergistic effects between targeted agents and conventional treatments or immunotherapies, aiming to enhance efficacy and overcome resistance mechanisms.
AI Impact Analysis on NUT Midline Carcinoma Treatment Market
User inquiries about AI's influence on the NUT Midline Carcinoma Treatment Market frequently center on its potential to revolutionize diagnosis, accelerate drug discovery, and personalize treatment strategies for this rare and aggressive cancer. Key themes include the anticipation of AI-powered diagnostic tools capable of rapidly and accurately identifying NUT fusions from pathological samples, the promise of AI in sifting through vast genomic and proteomic data to uncover novel therapeutic targets and biomarkers, and the expectation that AI will optimize patient selection for clinical trials and predict individual responses to specific therapies. Concerns often revolve around data privacy, the validation of AI algorithms in a rare disease context where data is scarce, and the integration of these complex technologies into existing clinical workflows. Users also question the ethical implications of AI-driven decisions and the need for robust regulatory oversight to ensure patient safety and equitable access to AI-enhanced care.
- Accelerated Diagnosis: AI algorithms can analyze pathology images and genomic data more rapidly and accurately than traditional methods, leading to earlier and more precise diagnosis of NUT Midline Carcinoma, crucial for initiating timely treatment.
- Enhanced Drug Discovery: AI models can predict potential drug candidates, simulate molecular interactions, and identify novel therapeutic targets by analyzing vast biological datasets, significantly shortening the drug development timeline for NMC-specific treatments.
- Personalized Treatment Planning: Machine learning can integrate patient-specific genomic, proteomic, and clinical data to recommend the most effective and least toxic treatment regimens, optimizing personalized medicine approaches for NMC patients.
- Biomarker Identification: AI can identify subtle patterns in patient data to discover new biomarkers for disease progression, treatment response, and resistance, providing critical insights for therapeutic monitoring and adjustment.
- Clinical Trial Optimization: AI can improve patient stratification for clinical trials, identify suitable candidates, and analyze trial outcomes more efficiently, thereby accelerating the development and approval of new NMC therapies.
- Real-world Evidence Generation: AI can process and interpret real-world data from electronic health records, enhancing post-market surveillance, identifying long-term treatment effects, and contributing to a deeper understanding of NMC patient cohorts.
- Improved Patient Monitoring: AI-powered remote monitoring systems can track patient progress, detect early signs of recurrence or adverse events, and enable proactive intervention, enhancing overall patient care and safety.
DRO & Impact Forces Of NUT Midline Carcinoma Treatment Market
The NUT Midline Carcinoma Treatment Market is shaped by a confluence of driving forces, significant restraints, and emerging opportunities, all interacting to create dynamic impact forces. A primary driver is the increasing understanding of the molecular pathogenesis of NMC, particularly the role of NUT fusion proteins, which has spurred targeted drug development. Advancements in diagnostic technologies, such as next-generation sequencing (NGS), enabling precise and early identification of these rare translocations, also significantly fuel market growth. Furthermore, the rising investment in oncology research and development, coupled with supportive regulatory frameworks that offer orphan drug designations and expedited approvals for rare diseases, provides a strong impetus for pharmaceutical companies to enter and innovate within this niche market. The growing adoption of personalized medicine approaches, where treatment is tailored to the patient's genetic profile, is another critical factor accelerating market expansion.
Despite these drivers, several restraints impede the market's full potential. The inherent rarity of NUT Midline Carcinoma leads to a very small patient population, posing challenges for clinical trial recruitment and making the economic viability of drug development difficult for some manufacturers. The high cost associated with advanced targeted therapies and molecular diagnostics, particularly in regions with less developed healthcare systems, acts as a significant barrier to access. Moreover, the aggressive nature of NMC and its propensity for rapid progression, often leading to late-stage diagnosis, limits the window for effective intervention. Treatment side effects and the development of drug resistance further complicate long-term management, necessitating continuous research into novel therapeutic strategies and combination regimens.
However, substantial opportunities exist to overcome these restraints and foster market growth. The increasing focus on biomarker discovery and development for better patient stratification and response prediction presents a lucrative avenue for innovation. The exploration of combination therapies, leveraging the synergistic effects of targeted agents, conventional treatments, and immunotherapies, offers promising pathways to improve efficacy and combat resistance. Furthermore, global collaborative efforts among researchers, clinicians, and industry stakeholders can enhance data sharing, accelerate clinical trials, and establish standardized treatment protocols. The growing awareness among healthcare professionals about rare cancers and the potential for early diagnosis through advanced molecular testing will also contribute significantly to expanding the market reach and improving patient outcomes. The ongoing development of novel drug delivery systems designed to enhance therapeutic efficacy and reduce systemic toxicity also represents a key area of opportunity.
- Drivers:
- Increasing understanding of NUT fusion biology leading to targeted therapy development.
- Advancements in molecular diagnostics (e.g., NGS) for precise NMC identification.
- Rising R&D investments in rare oncology diseases and orphan drug designations.
- Growing adoption of personalized and precision medicine approaches in cancer care.
- Urgent unmet medical need due to the aggressive nature and poor prognosis of NMC.
- Restraints:
- Extremely rare incidence of NUT Midline Carcinoma, limiting patient pool.
- High cost of advanced targeted therapies and molecular diagnostic tests.
- Challenges in clinical trial recruitment for a small and geographically dispersed patient population.
- Aggressive disease progression and late-stage diagnosis often reduce treatment efficacy.
- Development of drug resistance and significant side effects associated with treatments.
- Opportunities:
- Development of novel targeted therapies, particularly BET inhibitors and epigenetic modulators.
- Exploration of combination therapies to enhance efficacy and overcome resistance.
- Discovery and validation of new biomarkers for diagnosis, prognosis, and treatment response.
- Global collaborations to establish patient registries, standardize care, and accelerate research.
- Leveraging artificial intelligence and machine learning for drug discovery and personalized treatment.
- Impact Forces:
- Technological Advancements: Rapid evolution in genomics, proteomics, and drug discovery platforms.
- Regulatory Landscape: Orphan drug incentives, accelerated approval pathways, and evolving guidelines for rare cancers.
- Healthcare Expenditure: Influence of public and private funding on access to advanced diagnostics and treatments.
- Patient Advocacy: Growing patient and caregiver advocacy groups driving awareness and research funding.
- Competitive Intensity: Increasing number of biotech and pharmaceutical companies entering the rare oncology space.
Segmentation Analysis
The NUT Midline Carcinoma Treatment Market is segmented across various dimensions to provide a detailed understanding of its dynamics and growth opportunities. These segmentations typically include analyses by therapy type, drug class, end-user, and geographic region, reflecting the diverse approaches to managing this rare and aggressive cancer. The segmentation by therapy type differentiates between conventional treatments like chemotherapy and radiation therapy and more advanced, targeted approaches such as precision medicine and immunotherapy. This granular breakdown helps stakeholders understand the current market landscape, identify emerging trends in treatment modalities, and strategically position their products or services within the evolving therapeutic paradigm. The unique genetic signature of NMC heavily influences these segmentations, particularly in the drug class category, which emphasizes molecularly targeted agents.
Further segmentation by drug class is critical, given the specific molecular drivers of NMC. This segment typically focuses on BET inhibitors, which directly target the BRD4-NUT fusion protein, alongside other epigenetic modulators, kinase inhibitors, and conventional cytotoxic agents. Understanding the market share and growth trajectory of each drug class provides insights into the most effective and widely adopted pharmaceutical interventions. The end-user segmentation primarily categorizes the market into hospitals, specialized oncology clinics, and research institutions, reflecting where diagnosis and treatment initiation predominantly occur. This distinction is vital for drug manufacturers and medical device providers to tailor their marketing and distribution strategies effectively, ensuring their products reach the appropriate healthcare settings and practitioners.
Geographic segmentation is also paramount for a global market perspective, distinguishing between regions like North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. Each region presents unique market drivers, regulatory environments, and healthcare infrastructure, influencing the adoption and accessibility of NMC treatments. The comprehensive segmentation analysis not only delineates the current market structure but also projects future trends, highlighting areas of significant growth, competitive intensity, and potential for unmet needs. Such detailed analysis empowers pharmaceutical companies, healthcare providers, and investors to make informed decisions regarding R&D investment, market entry strategies, and resource allocation to optimize patient outcomes in this challenging oncology space.
- By Therapy Type:
- Targeted Therapy
- BET Inhibitors
- Epigenetic Modulators
- Kinase Inhibitors
- Other Targeted Agents
- Chemotherapy
- Radiation Therapy
- Immunotherapy
- Combination Therapies
- Targeted Therapy
- By Drug Class:
- Bromodomain and Extraterminal Domain (BET) Inhibitors
- Histone Deacetylase (HDAC) Inhibitors
- DNA Methyltransferase (DNMT) Inhibitors
- Other Epigenetic Modulators
- Cytotoxic Agents
- Immunological Checkpoint Inhibitors
- By End-User:
- Hospitals
- Specialized Oncology Clinics
- Academic & Research Institutions
- Ambulatory Surgical Centers
- By Region:
- North America (U.S., Canada)
- Europe (Germany, UK, France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, Japan, India, South Korea, Australia, Rest of Asia Pacific)
- Latin America (Brazil, Mexico, Argentina, Rest of Latin America)
- Middle East & Africa (South Africa, UAE, Saudi Arabia, Rest of MEA)
Value Chain Analysis For NUT Midline Carcinoma Treatment Market
The value chain for the NUT Midline Carcinoma Treatment Market begins with extensive upstream analysis, focusing on research and development (R&D) and the sourcing of raw materials. This initial stage is crucial for identifying novel drug targets, understanding the molecular mechanisms of NMC, and synthesizing potential therapeutic compounds. Pharmaceutical and biotechnology companies invest heavily in preclinical research, drug screening, and lead optimization, often collaborating with academic institutions and contract research organizations (CROs). The sourcing of high-quality chemical intermediates and biological components for drug manufacturing is also a critical upstream activity, requiring robust supply chain management and adherence to stringent quality standards to ensure product efficacy and safety. Patent protection and intellectual property management are paramount during this phase to safeguard innovations.
The midstream of the value chain involves the clinical development, manufacturing, and regulatory approval processes. Once potential drug candidates show promise in preclinical studies, they advance to rigorous clinical trials (Phases I, II, and III) to assess safety and efficacy in human subjects, particularly in a rare disease context where patient recruitment can be challenging. Following successful clinical trials, large-scale manufacturing of approved drugs takes place, often outsourced to contract manufacturing organizations (CMOs) or conducted in-house. This stage is followed by the complex process of obtaining regulatory approvals from authorities such as the FDA in the U.S. or EMA in Europe, which often involves specialized orphan drug designations and expedited review pathways for NMC treatments. Quality control and assurance are maintained throughout the manufacturing and approval processes to ensure compliance with global pharmaceutical standards.
Downstream analysis encompasses distribution, sales, and post-market activities. Approved NUT Midline Carcinoma treatments are distributed through a network of specialized channels, including pharmaceutical wholesalers, direct distribution to hospitals and oncology centers, and specialty pharmacies. Given the niche nature of NMC, direct sales and marketing efforts are often targeted at rare disease specialists, oncologists, and relevant healthcare institutions. Post-market surveillance, pharmacovigilance, and patient support programs are vital downstream activities, ensuring patient safety, monitoring long-term efficacy, and providing support services. The distribution channels can be both direct, where manufacturers engage directly with healthcare providers or large hospital systems, and indirect, involving third-party distributors and wholesalers to reach a broader network of clinics and pharmacies, especially in geographically dispersed markets. Effective communication and education for healthcare providers are also integral to ensuring appropriate usage and access to these specialized therapies.
NUT Midline Carcinoma Treatment Market Potential Customers
The primary potential customers for NUT Midline Carcinoma treatment products and services are healthcare providers and institutions specializing in oncology. This includes large university hospitals, comprehensive cancer centers, and specialized oncology clinics that possess the infrastructure and expertise to diagnose, treat, and manage rare and aggressive cancers. These institutions serve as the crucial point of care where patients with suspected or confirmed NMC receive multidisciplinary evaluation, molecular diagnostic testing, and therapeutic interventions. Their demand is driven by the need for effective, cutting-edge therapies that can improve patient outcomes in a disease characterized by poor prognosis and limited conventional treatment efficacy. The investment in advanced diagnostics, targeted therapeutics, and supportive care within these settings directly translates to their role as key purchasers in the market.
Another significant segment of potential customers includes research institutions and academic medical centers. These entities are not only involved in the clinical treatment of NMC but are also at the forefront of understanding its molecular biology, developing new diagnostic methods, and pioneering novel therapeutic strategies through basic and translational research. They represent a critical market for research-grade reagents, specialized laboratory equipment, and investigational drugs for clinical trials. Pharmaceutical and biotechnology companies also serve as potential customers, specifically in terms of in-licensing opportunities for promising drug candidates discovered by smaller biotech firms or academic labs, or through partnerships aimed at co-developing and commercializing innovative treatments for NMC. Their objective is to expand their oncology portfolios and address unmet medical needs within the rare cancer segment.
Ultimately, the end-users and beneficiaries of these products are the patients diagnosed with NUT Midline Carcinoma. While patients do not directly purchase the treatments in most healthcare systems, their medical needs drive the demand throughout the entire value chain. Healthcare payers, including government health programs, private insurance companies, and managed care organizations, also function as indirect customers. They influence market dynamics through their formulary decisions, reimbursement policies, and cost-effectiveness assessments of high-cost targeted therapies. Their role is to ensure access to necessary treatments while managing healthcare expenditures, making them critical stakeholders in the commercial viability and widespread adoption of NMC therapies. Advocacy groups and patient foundations, by raising awareness and funding research, also indirectly shape the market by influencing policy and R&D priorities.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 185 million |
| Market Forecast in 2032 | USD 340 million |
| Growth Rate | CAGR 9.2% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | EPZ-5676, GSK, F. Hoffmann-La Roche Ltd., Merck & Co., Inc., Novartis AG, Bristol-Myers Squibb Company, AstraZeneca plc, Pfizer Inc., Johnson & Johnson, Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company, Limited, Blueprint Medicines Corporation, Sanofi S.A., Eisai Co., Ltd., Seattle Genetics, Inc., MorphoSys AG, Loxo Oncology (Eli Lilly and Company), SpringWorks Therapeutics, Inc., Deciphera Pharmaceuticals, Inc., Array BioPharma Inc. (Pfizer). |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
NUT Midline Carcinoma Treatment Market Key Technology Landscape
The key technology landscape for the NUT Midline Carcinoma Treatment Market is rapidly evolving, driven by advancements in precision oncology and molecular diagnostics. Next-generation sequencing (NGS) stands as a foundational technology, enabling comprehensive genomic profiling of tumors to accurately identify NUT gene rearrangements, which is critical for diagnosis and therapeutic stratification. RNA sequencing and fluorescence in situ hybridization (FISH) are also vital for confirming these specific translocations. Beyond diagnostics, technologies like CRISPR/Cas9 gene editing are being explored in research settings to create cellular models of NMC, facilitating a deeper understanding of the disease's biology and enabling the testing of novel therapeutic strategies. These diagnostic and research tools are indispensable for both identifying the rare disease and advancing the science behind its treatment.
In terms of therapeutic technologies, the development of highly specific small molecule inhibitors forms a significant part of the landscape. Bromodomain and Extraterminal Domain (BET) inhibitors, for instance, are designed using advanced medicinal chemistry techniques to precisely block the activity of BRD4-NUT fusion proteins, thereby disrupting the oncogenic transcriptional program. Epigenetic modulators, another class of targeted drugs, leverage technologies that can reverse aberrant epigenetic marks caused by the NUT fusion, aiming to restore normal cellular differentiation. The development of these compounds relies on sophisticated drug design software, high-throughput screening platforms, and detailed structural biology insights to optimize their potency, selectivity, and pharmacokinetic profiles, ensuring they effectively target the disease without causing undue toxicity.
Furthermore, advanced drug delivery systems are being explored to enhance the efficacy and safety of NMC treatments. Nanotechnology-based drug delivery, for example, allows for improved drug targeting to tumor sites, reducing systemic exposure and minimizing off-target side effects. Immunotherapy platforms, while less established for NMC, also represent a significant technological frontier, including checkpoint inhibitors and adoptive cell therapies that aim to harness the patient's own immune system to fight the cancer. Bioinformatic tools and artificial intelligence are increasingly integrated across the entire technology landscape, from analyzing vast genomic data for biomarker discovery to predicting drug responses and optimizing clinical trial designs, further accelerating the development and implementation of cutting-edge treatments for NUT Midline Carcinoma.
Regional Highlights
- North America: This region holds a dominant share in the NUT Midline Carcinoma Treatment Market, attributed to its robust healthcare infrastructure, high R&D investments, advanced diagnostic capabilities, and a significant number of ongoing clinical trials. Favorable regulatory policies, including orphan drug designations from the FDA, incentivize pharmaceutical companies to develop and commercialize therapies for rare cancers. The presence of leading biopharmaceutical companies and well-established academic research centers further solidifies its market leadership, fostering rapid adoption of novel treatments and precision medicine approaches.
- Europe: Europe is another major market for NMC treatments, driven by strong government support for cancer research, increasing awareness among healthcare professionals, and the adoption of advanced molecular diagnostic techniques. Countries like Germany, the UK, and France are at the forefront of clinical research and therapeutic innovation. The European Medicines Agency (EMA) provides regulatory pathways that support the development of orphan drugs, stimulating investment. Collaborations between research institutes and industry players are common, enhancing the translation of scientific discoveries into clinical practice and expanding patient access to specialized care.
- Asia Pacific (APAC): The Asia Pacific region is projected to experience the fastest growth in the NMC treatment market due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness of rare cancers. Countries such as Japan, China, and India are investing heavily in oncology research and developing their capabilities in genomic sequencing and targeted therapies. While market penetration of advanced treatments is still growing, the large patient population and increasing prevalence of cancer, coupled with a growing focus on precision medicine, present significant opportunities for market expansion and new entrants.
- Latin America: The market in Latin America is in its nascent stage but shows promising growth potential. Factors contributing to this include expanding access to healthcare services, increasing diagnostic capabilities, and a rising focus on specialized oncology care in major economies like Brazil and Mexico. Challenges remain in terms of healthcare expenditure and regulatory complexities, but efforts to bridge the gap in rare cancer treatments are ongoing. International collaborations and local government initiatives are expected to drive gradual but steady market development.
- Middle East and Africa (MEA): The MEA region represents an emerging market for NUT Midline Carcinoma treatments, characterized by disparities in healthcare access and infrastructure. However, countries like the UAE and Saudi Arabia are making significant investments in healthcare modernization and advanced medical technologies, including oncology centers and molecular diagnostic labs. Increasing incidence of cancer and a growing emphasis on early diagnosis and personalized treatment are expected to fuel market growth. Partnerships with global pharmaceutical companies and technology transfer initiatives are crucial for improving access to specialized therapies in this region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the NUT Midline Carcinoma Treatment Market.- EPZ-5676
- GSK
- F. Hoffmann-La Roche Ltd.
- Merck & Co., Inc.
- Novartis AG
- Bristol-Myers Squibb Company
- AstraZeneca plc
- Pfizer Inc.
- Johnson & Johnson
- Takeda Pharmaceutical Company Limited
- Daiichi Sankyo Company, Limited
- Blueprint Medicines Corporation
- Sanofi S.A.
- Eisai Co., Ltd.
- Seattle Genetics, Inc.
- MorphoSys AG
- Loxo Oncology (Eli Lilly and Company)
- SpringWorks Therapeutics, Inc.
- Deciphera Pharmaceuticals, Inc.
- Array BioPharma Inc. (Pfizer)
Frequently Asked Questions
What is NUT Midline Carcinoma (NMC)?
NUT Midline Carcinoma is an extremely rare and aggressive cancer defined by chromosomal rearrangements involving the NUT gene, primarily forming BRD4-NUT fusion proteins. It typically arises in midline organs like the head, neck, and chest, characterized by rapid growth and resistance to conventional treatments. Early and accurate molecular diagnosis is crucial for effective management due to its distinct genetic signature.
What are the primary treatment options for NUT Midline Carcinoma?
Treatment for NUT Midline Carcinoma often involves a multidisciplinary approach combining conventional therapies such as chemotherapy and radiation, alongside surgical resection when feasible. Increasingly, targeted therapies, specifically Bromodomain and Extraterminal Domain (BET) inhibitors, are becoming central to treatment due to their ability to block the oncogenic NUT fusion protein. Immunotherapy and epigenetic modulators are also being explored in clinical trials to enhance patient outcomes.
How is NUT Midline Carcinoma diagnosed?
Diagnosis of NUT Midline Carcinoma relies on advanced molecular testing methods. These include immunohistochemistry (IHC) for NUT protein expression, fluorescence in situ hybridization (FISH) to detect NUT gene rearrangements, and next-generation sequencing (NGS) to identify specific NUT fusion partners. These precise diagnostic techniques are essential to confirm the presence of this rare genetic signature and differentiate NMC from other poorly differentiated carcinomas, guiding targeted therapeutic decisions.
What are the current challenges in treating NUT Midline Carcinoma?
Significant challenges in treating NUT Midline Carcinoma include its extreme rarity, which makes patient recruitment for clinical trials difficult, and its aggressive nature, leading to rapid progression and often late-stage diagnosis. Furthermore, the high cost of specialized targeted therapies and molecular diagnostics, coupled with the potential for drug resistance and side effects, poses hurdles for patient access and sustained efficacy. Limited awareness among general practitioners also contributes to diagnostic delays.
What is the role of targeted therapies like BET inhibitors in NMC treatment?
Targeted therapies, especially BET inhibitors, play a pivotal role in NMC treatment by directly disrupting the oncogenic activity of the BRD4-NUT fusion protein. These inhibitors interfere with the epigenetic machinery that drives tumor growth, leading to tumor regression and differentiation in preclinical and clinical studies. They offer a more precise treatment approach compared to traditional chemotherapy, aiming to improve efficacy and reduce systemic toxicity by targeting the specific genetic driver of the disease.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
