Oral Sleep Apnea Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 431176 | Date : Nov, 2025 | Pages : 241 | Region : Global | Publisher : MRU
Oral Sleep Apnea Devices Market Size
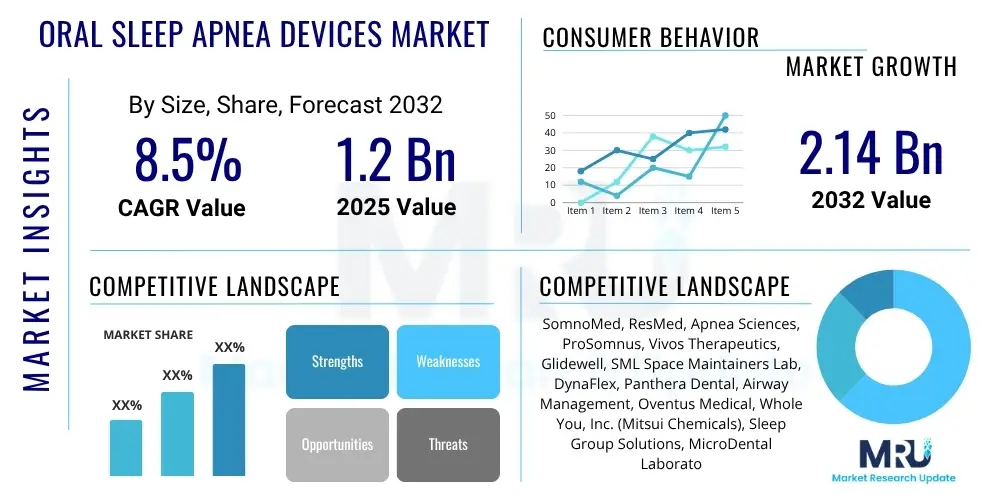
The Oral Sleep Apnea Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2025 and 2032. The market is estimated at USD 1.2 Billion in 2025 and is projected to reach USD 2.14 Billion by the end of the forecast period in 2032.
Oral Sleep Apnea Devices Market introduction
The Oral Sleep Apnea Devices market signifies a rapidly advancing and strategically crucial sector within the broader medical technology and dental sleep medicine industries. It is dedicated to delivering innovative, non-pharmacological, and non-surgical solutions for the management of sleep-disordered breathing, most notably obstructive sleep apnea (OSA) and pervasive primary snoring. These sophisticated devices are meticulously engineered as custom-fitted intraoral appliances, designed for comfortable and effective use during sleep. Their primary therapeutic mechanism revolves around precisely repositioning the lower jaw, or mandible, and/or maintaining the tongue in a forward position. This biomechanical intervention is critical in preventing the posterior collapse of soft tissues in the pharyngeal airway, which is the underlying cause of recurrent airway obstructions, resulting in apnea episodes and disruptive snoring. By maintaining an open and stable airway throughout the night, these devices aim to restore normal breathing patterns, thereby mitigating the profound health and quality-of-life impacts associated with sleep fragmentation and intermittent hypoxia.
The product portfolio within this dynamic market primarily comprises two principal categories: Mandibular Advancement Devices (MADs) and Tongue Retaining Devices (TRDs). Mandibular Advancement Devices constitute the larger and more widely adopted segment, functioning by gently yet firmly advancing the mandible and its associated soft tissues, including the tongue, forward. This forward repositioning significantly expands the posterior airway space, encompassing both the retroglossal and retropalatal regions, thus reducing the probability of inspiratory airflow limitation and collapse. In contrast, Tongue Retaining Devices operate by utilizing a gentle suction mechanism to hold the tongue in an anteriorly protruded position, effectively preventing it from retracting and obstructing the airway. These devices find extensive application in the treatment of mild to moderate obstructive sleep apnea, offering a highly valuable and often preferred alternative for patients diagnosed with severe OSA who experience intolerance, discomfort, or non-adherence to Continuous Positive Airway Pressure (CPAP) therapy, which is often considered the first-line treatment. Furthermore, they provide a reliable and efficacious solution for individuals whose primary complaint is disruptive snoring, often leading to social and relationship strain. The multifaceted benefits of these oral appliances are profound, encompassing significantly improved sleep architecture and quality, a substantial reduction or elimination of snoring, decreased excessive daytime sleepiness and chronic fatigue, enhanced cognitive function, and crucially, offering a non-surgical, reversible, and generally more patient-friendly therapeutic pathway compared to other interventional options. The ability to customize these devices for an optimal fit further enhances their clinical efficacy and user acceptance.
The sustained and vigorous expansion of the Oral Sleep Apnea Devices market is intrinsically linked to several compelling factors. A paramount driver is the alarming escalating global prevalence of obstructive sleep apnea, a condition whose profound systemic health consequences, including increased risks of cardiovascular disease, metabolic regulation, and overall quality of life, are becoming increasingly recognized by both the medical community and the general public. This heightened awareness inevitably translates into a surge in diagnostic efforts and a subsequent increase in the demand for effective therapeutic interventions. Critically, a substantial segment of patients prescribed CPAP therapy, despite its proven efficacy, experience challenges with comfort, claustrophobia, noise, mask fit, or logistical inconveniences, leading to high rates of non-adherence. This crucial segment actively seeks more tolerable, discreet, and convenient treatment alternatives, positioning oral appliances as an ideal solution. Moreover, continuous advancements in materials science, particularly the development of biocompatible and durable polymers, combined with sophisticated digital dentistry technologies such as intraoral scanning, 3D printing, and CAD/CAM software, have revolutionized device design and manufacturing. These technological leaps enable the production of oral appliances that offer superior comfort, enhanced precision of fit, greater durability, and optimized therapeutic effectiveness, directly leading to improved patient satisfaction and long-term compliance. Lastly, the inexorable trend of an aging global demographic, which is inherently more susceptible to developing sleep-disordered breathing conditions, further underpins the rising demand for accessible, comfortable, and efficacious sleep apnea management tools, assuring a robust market outlook.
Oral Sleep Apnea Devices Market Executive Summary
The Oral Sleep Apnea Devices market is currently navigating a period of significant growth and innovation, shaped by several dynamic business trends designed to optimize market reach, enhance product performance, and improve patient engagement. A prominent trend involves an intensified focus on strategic collaborations, encompassing joint ventures, licensing agreements, and mergers and acquisitions among manufacturers, dental laboratories, and even pharmaceutical companies. These concerted efforts are primarily aimed at consolidating market share, diversifying product portfolios to offer a more comprehensive suite of solutions, and pooling research and development capabilities to accelerate product innovation. Furthermore, there is a discernible and accelerating shift towards leveraging digital platforms for both product distribution and patient engagement. This includes the expansion of sophisticated direct-to-consumer (D2C) models for certain types of non-custom or semi-custom oral appliances, frequently augmented by integrated telehealth services that facilitate remote consultations and ongoing support. This approach democratizes access for consumers, particularly for primary snoring and mild OSA, catering to a growing demand for convenient, discreet, and personalized healthcare solutions. Concurrently, substantial investments are being made in advanced material science and manufacturing processes, specifically in developing biocompatible, lightweight, and highly durable polymers, coupled with the widespread adoption of precision 3D printing technologies. These innovations are revolutionizing device fabrication, enabling unparalleled levels of customization, significantly improving patient comfort, and crucially, boosting long-term adherence rates, which are paramount for successful therapeutic outcomes. Competitive strategies are increasingly centering on differentiating products through enhanced patient experience, clinical validation, and ease of professional integration.
From a geographical perspective, the market exhibits distinct regional dynamics. North America and Europe collectively represent the dominant market shares, a position attributable to several deeply entrenched factors. These include a high level of public and professional awareness regarding the adverse health consequences of sleep disorders, highly developed and extensive healthcare infrastructures replete with specialized sleep clinics and dental sleep medicine practices, and generally favorable reimbursement policies that alleviate a substantial portion of the financial burden on patients. These regions also benefit from established clinical guidelines and a mature ecosystem for the integration of oral appliance therapy into routine medical and dental practice. In stark contrast, the Asia Pacific (APAC) region is forecasted to be the fastest-growing market globally. This exponential growth is underpinned by the presence of a vast, largely undiagnosed patient population, representing immense untapped potential, coupled with rapid and continuous improvements in healthcare accessibility and significant increases in healthcare expenditure across key economies such as China, India, and Japan. Governments in these nations are increasingly prioritizing public health initiatives, including those targeting sleep health, which translates into greater investment in diagnostic capabilities and diverse treatment options. Similarly, nascent but promising growth trajectories are observed in Latin America, the Middle East, and Africa, as healthcare systems in these regions develop and as awareness pertaining to sleep disorders gradually permeates broader societal consciousness.
An in-depth analysis of market segmentation trends further illuminates key areas of expansion and preference. Mandibular Advancement Devices (MADs) continue to hold the largest share within the product type segment, primarily owing to their extensive clinical validation, broad acceptance among both dental and sleep medicine practitioners, and their versatility in treating various severities of OSA. Within the MADs category, the sub-segment of custom-fitted devices is poised for superior growth and market penetration. This trajectory is a direct response to the escalating demand for highly personalized treatment solutions that guarantee an optimal fit, maximum anatomical conformity, and tailored therapeutic effect—all critical determinants of long-term patient adherence and successful clinical outcomes. End-user adoption patterns reveal a steady increase in the utilization of oral appliances across specialized sleep clinics and general dental practices, and particularly within dental sleep medicine practices, which are becoming pivotal referral hubs. Concurrently, a burgeoning interest and infrastructural support are noted for the utilization of these devices in home care settings, reflecting a growing patient preference for convenience, self-management, and the ability to integrate treatment seamlessly into daily life, where clinically appropriate. The ongoing and rapid integration of advanced digital dentistry techniques, such as high-resolution intraoral scanning, sophisticated CAD/CAM software, and precision 3D printing, is fundamentally transforming the entire manufacturing and fitting processes of these devices, significantly enhancing precision, efficiency, and overall clinical effectiveness, thereby driving segment innovation.
AI Impact Analysis on Oral Sleep Apnea Devices Market
Common user inquiries regarding the influence of artificial intelligence (AI) on the Oral Sleep Apnea Devices market frequently revolve around its potential to revolutionize the entire patient journey, from initial screening and precise diagnosis to highly personalized treatment planning, streamlined device fabrication, and continuous remote monitoring. Users are keenly interested in whether AI can offer more precise and less invasive methods for identifying suitable candidates for oral appliance therapy, optimize the design parameters of devices to perfectly match individual patient anatomies, and implement smart monitoring solutions to track usage and treatment effectiveness in real-time. Underlying themes include the promise of improved patient outcomes, increased comfort, and greater accessibility, alongside concerns regarding data privacy, the reliability of AI-driven clinical recommendations, and the potential for technological disparities or increased costs associated with integrating such advanced technological infrastructures. Nevertheless, there is a strong collective expectation that AI will usher in an unprecedented era of highly personalized, efficient, and accessible sleep apnea management, moving beyond generic, one-size-fits-all approaches to deeply customized therapeutic strategies tailored to individual patient anatomies, physiologies, and lifestyles, particularly in areas like predictive modeling for treatment success and automated adjustments for optimal device efficacy and patient comfort. The continuous development of robust, explainable AI models is crucial for building trust and ensuring widespread adoption.
- Enhanced Diagnostic Capabilities: AI algorithms are being meticulously developed and continuously refined to analyze a vast array of complex physiological data, including inputs from polysomnography, advanced home sleep tests, and anthropometric measurements such as detailed facial and intraoral 3D scans. These sophisticated AI-powered tools can identify subtle, often imperceptible patterns, and biomarkers indicative of OSA severity, predict the likelihood of treatment success with oral appliances, and accurately categorize patients who would benefit most from this therapy, thus significantly improving diagnostic precision and reducing the time from suspicion to effective intervention. This also encompasses the advanced analysis of complex airway morphology and dynamic changes that occur during various sleep stages.
- Personalized Device Design and Manufacturing: The profound integration of AI with state-of-the-art Computer-Aided Design (CAD) and Computer-Aided Manufacturing (CAM) systems is fundamentally transforming the creation of oral sleep apnea devices. AI algorithms are engineered to process high-resolution 3D intraoral scans, CBCT (Cone Beam Computed Tomography) data, and other anatomical information to generate exquisitely customized mandibular advancement devices. These intelligent systems can simulate numerous jaw positions and movements to identify the precise, optimal therapeutic advancement for each patient, accounting for their unique anatomical features, dental occlusion, and specific biomechanical requirements. This results in devices that not only offer an unparalleled anatomical fit but also maximize therapeutic effect by precisely controlling airway opening and stability, thereby dramatically improving patient comfort, minimizing potential side effects, and crucially boosting long-term adherence rates while significantly reducing the need for extensive post-fitting manual adjustments.
- Improved Treatment Planning and Remote Monitoring: AI tools are becoming increasingly instrumental in assisting clinicians with highly individualized treatment planning, leveraging sophisticated predictive analytics built upon extensive datasets of patient demographics, detailed physiological parameters, and historical treatment responses. Beyond initial planning, advanced AI-driven remote monitoring solutions are rapidly emerging. These integrate miniature, unobtrusive sensors directly within oral appliances or via companion peripheral devices. These sensors are designed to continuously track critical parameters such as actual device usage time, precise jaw position throughout the night, and even subtle indicators of sleep quality and breathing patterns. AI processes this vast stream of real-time data, generating actionable insights and providing instant feedback and alerts to both patients and healthcare providers. This facilitates proactive intervention, allows for dynamic and timely adjustments to device settings, and provides objective, irrefutable evidence of treatment adherence and efficacy. Such capabilities are revolutionizing overall patient management, fostering continuous optimization of therapy, and ultimately leading to significantly improved therapeutic outcomes.
- Predictive Analytics for Outcomes and Risk Stratification: Robust AI models are being meticulously trained on massive, anonymized datasets encompassing a wide array of patient demographics, comprehensive medical histories, intricate anatomical features, and diverse treatment outcomes from large patient cohorts. This extensive training enables AI to predict with escalating accuracy the probability of successful treatment for oral appliance therapy in specific individuals, or conversely, to precisely identify patients who may be at a higher inherent risk of treatment failure, non-adherence, or the development of adverse effects. This powerful predictive capability empowers clinicians to make more data-driven and informed decisions regarding optimal treatment selection, allowing for a truly personalized approach from the outset. It also facilitates effective management of patient expectations and aids significantly in stratifying patient risk profiles for long-term health complications often associated with chronic, untreated obstructive sleep apnea, leading to more targeted and efficient care.
- Supply Chain Optimization and Operational Efficiency: At a broader, industry-wide operational level, advanced AI algorithms are being strategically deployed to optimize the entire supply chain for oral sleep apnea devices. This includes highly accurate forecasting of demand for specific materials, device types, and components, intelligent inventory management systems designed to minimize waste and ensure the consistent and timely availability of all necessary resources, and the streamlined optimization of complex manufacturing workflows. AI can precisely identify potential bottlenecks in production lines, optimize resource allocation (including human capital and machinery), and significantly enhance quality control processes through automated defect detection. This leads to dramatically faster production cycles, considerable reductions in manufacturing costs, and ultimately, enhanced accessibility of these vital medical devices to a wider patient population through significantly improved overall operational efficiency and responsiveness across the entire industry ecosystem. Furthermore, AI can assist in compliance with evolving regulatory standards by tracking material origins and production parameters.
DRO & Impact Forces Of Oral Sleep Apnea Devices Market
The Oral Sleep Apnea Devices market operates within a dynamic and multifaceted environment, profoundly influenced by a complex interplay of powerful driving forces, intrinsic restraints, and promising opportunities, all of which are paramount for comprehensive strategic market analysis and forecasting. A primary and undeniably potent driver is the alarming and globally escalating prevalence of obstructive sleep apnea (OSA). This pervasive chronic condition, though frequently underdiagnosed, is now widely recognized for its severe and multifarious systemic health comorbidities, including a heightened risk of cardiovascular disease, resistant hypertension, stroke, type 2 diabetes, metabolic syndrome, and significant neurocognitive impairments. This mounting epidemiological burden is relentlessly fueling the demand for efficacious, patient-centric therapeutic interventions. Complementing this, a critical factor driving market adoption is the well-documented challenge of long-term adherence to Continuous Positive Airway Pressure (CPAP) therapy, which, despite being the established gold standard for moderate to severe OSA, often presents significant hurdles such as mask discomfort, claustrophobia, nasal irritation, noise, or logistical inconveniences. A substantial segment of patients actively seeks more tolerable, discreet, and convenient alternatives, thereby positioning oral appliance therapy as a highly attractive and clinically viable solution. Furthermore, the relentless pace of technological advancements in materials science, leading to the development of advanced biocompatible polymers, coupled with sophisticated digital dentistry technologies like 3D printing and CAD/CAM systems, is fundamentally transforming device design and manufacturing. These innovations facilitate the creation of oral appliances that are not only more comfortable and durable but also exquisitely customized to individual patient anatomies, crucially enhancing patient acceptance, long-term compliance, and overall clinical efficacy. The pervasive and increasing public and professional awareness regarding the insidious health risks associated with untreated sleep apnea also acts as a powerful catalyst, stimulating proactive diagnostic efforts and encouraging treatment-seeking behaviors across diverse demographics.
Despite these significant propelling forces, the market concurrently faces several persistent and impactful restraints that modulate its potential for accelerated growth and wider adoption. Foremost among these are the often substantial out-of-pocket costs associated with obtaining high-quality, custom-fitted oral appliances. This financial burden can represent a significant barrier to access for numerous patients, particularly in healthcare systems where insurance coverage is fragmented or non-existent, or in regions with lower disposable incomes. The situation is further complicated by variable and frequently insufficient reimbursement policies across different private and public insurance providers and geographical territories, creating stark disparities in access to this effective form of therapy. A critical restraint also originates from a lingering lack of widespread awareness and comprehensive education among general medical practitioners, who may not be fully informed about oral appliance therapy as a clinically validated and effective treatment option for mild to moderate OSA, or as a viable alternative for CPAP-intolerant patients. This knowledge gap often results in suboptimal referral rates to qualified dental sleep specialists. Historically, a perception, although increasingly challenged by accumulating robust clinical evidence, has existed that oral appliances are inherently less efficacious than CPAP, especially for more severe cases of OSA. This perception continues to influence some clinical decision-making and patient preferences, despite growing evidence supporting their effectiveness in specific patient populations. Furthermore, the inherent necessity for specialized dental expertise for accurate diagnosis, precise fitting, titration, and diligent ongoing follow-up care for oral appliances significantly limits their accessibility in geographically underserved or remote areas where such specialized professionals are scarce. Complex and often evolving regulatory hurdles, which dictate stringent approval processes, manufacturing standards, and clinical guidelines, can also introduce considerable delays, increase research and development costs for manufacturers, and impact market entry and expansion strategies.
Notwithstanding these considerable challenges, the market is fertile ground for numerous compelling opportunities that promise significant future growth, technological innovation, and expanded patient access. The vast, largely untapped patient populations residing in emerging economies, particularly across the Asia Pacific region and Latin America, represent an immense market potential. As healthcare infrastructure continues to improve, and as awareness of sleep disorders and their treatments increases in these regions, the demand for accessible and effective oral appliances is expected to surge. The burgeoning expansion and sophisticated refinement of direct-to-consumer (D2C) sales models, particularly for certain types of non-custom or semi-custom snoring and mild OSA devices, often seamlessly integrated with advanced telehealth consultations, are pivotal in democratizing patient access and offering unparalleled convenience. These models circumvent traditional healthcare bottlenecks, reaching a broader consumer base. Strategic collaborations and the development of integrated care pathways, fostering stronger partnerships between oral appliance manufacturers, dental professionals, sleep physicians, and primary care providers, are crucial in establishing comprehensive patient journeys from initial screening and diagnosis through long-term therapeutic management, thereby significantly improving treatment uptake, adherence, and outcomes. Moreover, continuous, aggressive research and development efforts are unlocking novel material innovations, enabling the creation of even more comfortable, durable, discreet, and lightweight devices with superior biomechanical properties. The rapidly advancing field of smart oral appliances, equipped with integrated micro-sensors for objective adherence monitoring, positional feedback, and even subtle physiological tracking, presents a transformative opportunity for enhancing patient compliance and facilitating proactive, data-driven adjustments to therapy. The broader societal emphasis on preventative healthcare, wellness initiatives, and improved quality of life further positions oral appliances as an exceptionally attractive solution for addressing primary snoring and mild to moderate OSA at earlier stages, potentially preventing disease progression and significantly improving overall public health and individual well-being.
Segmentation Analysis
The Oral Sleep Apnea Devices market is meticulously delineated through a comprehensive segmentation structure, which is absolutely indispensable for gaining a nuanced and granular understanding of its diverse constituent components and the intricate market dynamics that collectively drive its evolution and growth. This detailed analytical framework empowers all stakeholders, including innovative manufacturers, efficient distributors, and dedicated healthcare providers, with invaluable insights into specific product categories that are gaining traction, evolving end-user preferences, and the most efficacious distribution strategies tailored to distinct market demographics. By systematically dissecting the market along these various critical dimensions—product type, material, end-user, and distribution channel—businesses are uniquely positioned to formulate highly targeted and impactful marketing campaigns. This segmentation also facilitates the strategic refinement of product development roadmaps to precisely address identified unmet patient needs and emerging clinical requirements, and enables the optimal allocation of resources to capitalize on high-growth segments and nascent opportunities. Such a granular and data-driven analytical approach is utterly indispensable for effectively navigating the competitive landscape, identifying lucrative niche markets, and proactively understanding the continually evolving demands of both the patient population and the broader clinical community. This ensures informed decision-making, fosters sustained innovation, and ultimately drives robust, resilient, and ethical growth within the oral sleep apnea device industry, contributing to better patient outcomes globally.
- By Product Type
- Mandibular Advancement Devices (MADs): These are the most prevalent and widely prescribed oral appliances. MADs are custom-fabricated devices designed to gently advance the mandible (lower jaw) and consequently the tongue forward, increasing the space in the pharynx and thereby preventing the collapse of airway soft tissues during sleep. They are highly effective for mild to moderate OSA and are available in both custom-fitted and semi-custom/boil-and-bite configurations, with custom variants offering superior fit and efficacy.
- Tongue Retaining Devices (TRDs): Less common than MADs but clinically significant, TRDs function by holding the tongue in a protruded, anterior position using a small, soft suction bulb. This mechanism prevents the tongue from retracting posteriorly and obstructing the airway. TRDs are primarily considered for patients who find MADs uncomfortable, have specific dental anatomical limitations, or suffer from conditions such as severe temporomandibular joint disorder (TMJD) which might contraindicate MAD usage.
- By Material
- Acrylic: Widely utilized, particularly in the fabrication of custom-made devices, due to its exceptional durability, rigidity, and stability. Acrylic allows for precise molding and intricate adjustments by dental professionals, ensuring a bespoke fit. Its biocompatibility and longevity make it a preferred material for high-quality, long-term use appliances.
- Thermoplastic: This category encompasses materials that become pliable when heated and rigid upon cooling. They are commonly found in over-the-counter or semi-custom boil-and-bite devices, which can be softened in hot water and molded by the patient to their dentition for a personalized, albeit less precise, fit. Advanced thermoplastics are also increasingly employed in custom devices for enhanced comfort and flexibility.
- Others: This segment includes innovative and newer biocompatible polymers, such as various types of nylon, specialized silicones, and advanced multi-layered composites. These materials are meticulously engineered for enhanced flexibility, superior comfort, reduced bulk, improved longevity, and sometimes hypoallergenic properties, addressing specific patient needs and driving continuous product evolution.
- By End-User
- Hospitals: While not typically the primary site for device fitting, hospitals play a crucial role in the initial diagnosis of OSA and often refer patients to specialized sleep clinics or dental practices for oral appliance therapy. They may also participate in research or manage severe cases requiring integrated care pathways.
- Sleep Clinics and Dental Practices: These represent the most significant points of care for the prescription, fitting, titration, and ongoing management of oral sleep apnea devices. Specialized dental practices with a focus on dental sleep medicine are particularly critical, as the therapy fundamentally involves dental expertise for optimal outcomes.
- Home Care Settings: Driven by advancements in user-friendly device designs and the proliferation of telehealth services, patients can increasingly manage their oral appliance therapy within their home environment. This includes self-monitoring, remote consultations, and follow-up care from healthcare professionals, enhancing convenience and adherence for suitable candidates.
- By Distribution Channel
- Direct Sales: This channel involves manufacturers selling their products directly to large institutional buyers, such as major hospital networks, university dental schools, or high-volume specialized sleep clinics. This allows manufacturers greater control over branding, pricing strategies, and fosters direct, strong customer relationships.
- Third-Party Distributors: These entities play a vital role in providing extensive market reach, distributing oral sleep apnea devices to a broader, more fragmented network of smaller independent dental practices, regional hospitals, and local pharmacies. Distributors leverage their existing logistical infrastructure, sales teams, and established client relationships to ensure widespread product availability.
- Online Pharmacies and Retailers: This is a rapidly expanding channel, particularly for over-the-counter or semi-custom devices designed for snoring or very mild OSA. It offers unparalleled convenience, often competitive pricing, and accessibility to a wider consumer base, frequently complemented by online consultations or virtual fitting guidance.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 1.2 Billion |
| Market Forecast in 2032 | USD 2.14 Billion |
| Growth Rate | 8.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | SomnoMed, ResMed, Apnea Sciences, ProSomnus, Vivos Therapeutics, Glidewell, SML Space Maintainers Lab, DynaFlex, Panthera Dental, Airway Management, Oventus Medical, Whole You, Inc. (Mitsui Chemicals), Sleep Group Solutions, MicroDental Laboratories, Strong Dental, The Snore Guard, ZQuiet, SnoreRx, Silent Nite, Moses Appliance, aveoTSD, SurgiTel, NightLase (Fotona), CustMbite, Sleep Guard, Oral Arts Dental Laboratory, Belo Medical Group |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Oral Sleep Apnea Devices Market Key Technology Landscape
The Oral Sleep Apnea Devices market is experiencing a profound and continuous technological transformation, driven by an unwavering commitment to enhancing therapeutic efficacy, significantly improving patient comfort, and substantially increasing overall accessibility. At the vanguard of this dynamic evolution is the pervasive and increasingly sophisticated adoption of 3D printing (additive manufacturing), intricately combined with state-of-the-art Computer-Aided Design (CAD) and Computer-Aided Manufacturing (CAM) systems. This integrated digital workflow represents a fundamental paradigm shift from conventional, labor-intensive, and often less precise impression-based fabrication methods. The process initiates with high-resolution digital intraoral scanning, which meticulously captures an exact three-dimensional digital replica of the patient's dentition, oral soft tissues, and surrounding anatomical structures. This precise data is then seamlessly imported into advanced CAD software, where highly skilled dental technicians and specialists can digitally design the oral appliance, perform intricate anatomical adjustments, simulate various mandibular advancement positions, and optimize the device architecture for maximum therapeutic effect while concurrently ensuring unparalleled patient comfort and minimizing bulk. The finalized digital design is then transmitted to specialized 3D printers, which fabricate the device layer by precise layer, utilizing a range of medical-grade biocompatible resins, advanced polymers, or other specialized materials. This synergistic technological approach dramatically improves the accuracy of fit, drastically reduces chair time for dental professionals during fitting appointments, minimizes material waste, and facilitates rapid iterative design modifications, all directly contributing to superior patient outcomes, enhanced long-term adherence, and an overall more streamlined and efficient manufacturing process. The ability to create highly individualized, patient-specific devices ensures optimal therapeutic benefit and vastly improves the user experience, making compliance more likely.
Regional Highlights
- North America: This region consistently retains its position as the largest and most influential market for Oral Sleep Apnea Devices, underpinned by an exceptionally high prevalence of diagnosed Obstructive Sleep Apnea (OSA) across its population, coupled with robust healthcare expenditure and a highly sophisticated and extensive healthcare infrastructure. The market in North America benefits substantially from well-established and generally favorable reimbursement policies, which significantly mitigate out-of-pocket costs for patients, thereby stimulating higher adoption rates. Furthermore, the presence of vigorous public and professional awareness campaigns regarding sleep disorders, combined with the strong foothold of numerous leading market players and a mature ecosystem of advanced dental sleep medicine practices, collectively contribute to the region's enduring market dominance, continuous innovation, and leadership in therapeutic advancements.
- Europe: The European market for oral sleep apnea devices demonstrates a trajectory of steady, consistent, and resilient growth. This sustained expansion is firmly supported by a progressively aging population that exhibits an inherently higher susceptibility to various sleep disorders, alongside a continually increasing awareness level among both medical professionals and the general public concerning the critical health implications of untreated sleep apnea. Favorable and often progressive regulatory environments in key European nations, which actively support and integrate oral appliance therapy into standard clinical practice guidelines, coupled with continuous improvements in healthcare access and the widespread adoption of advanced digital dental technologies across the continent, are crucial and synergistic factors propelling significant market expansion and wider clinical acceptance.
- Asia Pacific (APAC): The APAC region is definitively projected to be the fastest-growing market globally for Oral Sleep Apnea Devices, signifying immense future potential. This accelerated growth is fundamentally attributed to its vast, largely undiagnosed patient population, which represents an enormous untapped market. This is coupled with rapid and substantial improvements in healthcare infrastructure, increasing governmental investment in public health initiatives (including sleep health programs), and rapidly rising disposable incomes across major economies such as China, India, Japan, and Australia. These factors are collectively leading to greater accessibility and widespread adoption of advanced sleep apnea diagnostic capabilities and diverse treatment options, including sophisticated custom-fitted oral appliances, marking a significant shift in regional healthcare priorities.
- Latin America: This region presents compelling emerging opportunities within the Oral Sleep Apnea Devices market, characterized by evolving and modernizing healthcare systems and a gradually but consistently increasing understanding and recognition of sleep-disordered breathing among both the clinical community and the general populace. While the current market penetration and per capita expenditure on sleep apnea devices remain comparatively lower than in highly developed regions, increasing foreign and domestic investments in medical infrastructure, coupled with the expansion of a middle-class population demanding better and more accessible healthcare services, are strongly anticipated to significantly drive the demand and adoption of oral sleep apnea devices throughout the forecast period, positioning it for substantial future growth.
- Middle East and Africa (MEA): The MEA market for oral sleep apnea devices is experiencing a gradual but distinctly discernible expansion. This growth is largely fueled by improving access to healthcare services, particularly in burgeoning urban centers, increasing health awareness campaigns targeting lifestyle-related diseases (including sleep apnea), and the broader demographic trend of urbanization. Government-led initiatives and strategic investments aimed at upgrading and modernizing healthcare infrastructure across various countries in the region also contribute positively to market growth, despite it currently representing a relatively smaller market share when compared to the more mature and established regions of North America and Europe. The focus on improving public health and diversifying healthcare offerings is a key driver.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Oral Sleep Apnea Devices Market.- SomnoMed
- ResMed
- Apnea Sciences
- ProSomnus
- Vivos Therapeutics
- Glidewell
- SML Space Maintainers Lab
- DynaFlex
- Panthera Dental
- Airway Management
- Oventus Medical
- Whole You, Inc. (Mitsui Chemicals)
- Sleep Group Solutions
- MicroDental Laboratories
- Strong Dental
- The Snore Guard
- ZQuiet
- SnoreRx
- Silent Nite
- Moses Appliance
- aveoTSD (Tongue Stabilizing Device)
- SurgiTel
- NightLase (Fotona)
- CustMbite
- Sleep Guard
- Oral Arts Dental Laboratory
- Belo Medical Group
Frequently Asked Questions
What is an oral sleep apnea device and how does it fundamentally function?
An oral sleep apnea device is a highly specialized, custom-fitted, and removable dental appliance precisely designed to be comfortably worn in the mouth exclusively during sleep. Its fundamental therapeutic mechanism involves gently, yet effectively, repositioning the lower jaw (mandible) and/or stabilizing the tongue in a forward position. This strategic anatomical adjustment is crucial in preventing the posterior collapse of the soft tissues in the pharyngeal region of the throat. By maintaining an open and stable airway throughout the nocturnal period, this action meticulously mitigates chronic snoring and significantly reduces both the frequency and severity of breathing interruptions definitively associated with mild to moderate obstructive sleep apnea (OSA). These devices represent a comfortable, often preferred, and clinically validated alternative to Continuous Positive Airway Pressure (CPAP) therapy for a significant portion of suitable candidates, offering a less intrusive and more discreet treatment experience.
What are the key efficacy rates and primary benefits associated with using oral sleep apnea devices?
Oral sleep apnea devices demonstrate substantial and clinically proven efficacy, particularly for individuals with mild to moderate OSA and those experiencing persistent, disruptive primary snoring. Their use leads to significant and measurable improvements in overall sleep quality, a marked reduction in snoring volume and frequency, and consequently, a decrease in excessive daytime sleepiness and chronic fatigue. Patients often report enhanced cognitive function, improved mood, and a significantly elevated overall quality of life. For many appropriate patients, custom-fitted oral appliances offer a level of therapeutic effectiveness highly comparable to CPAP therapy within their specific indications. Crucially, this effectiveness is often achieved with superior patient comfort, enhanced portability, and notably higher long-term adherence rates, making them a highly attractive and patient-centric treatment option that fosters sustained compliance and better health outcomes.
Who is typically identified as a suitable candidate for oral sleep apnea device therapy?
Generally, suitable candidates for oral sleep apnea device therapy predominantly include individuals who have received a formal medical diagnosis of mild to moderate obstructive sleep apnea. They are also considered an excellent and often essential therapeutic option for patients diagnosed with severe OSA who have unequivocally demonstrated an inability to tolerate or consistently comply with Continuous Positive Airway Pressure (CPAP) therapy due to persistent discomfort, severe claustrophobia, mask fit issues, or other significant challenges. Additionally, individuals experiencing problematic primary snoring, even in the absence of formally diagnosed apnea, can derive substantial benefit from these devices. A comprehensive and meticulous evaluation conducted by a qualified dental sleep medicine specialist, often in close collaboration with a board-certified sleep physician, is absolutely essential to accurately determine individualized suitability, rule out contraindications, and ensure optimal, personalized treatment planning for each patient.
Are oral sleep apnea devices typically covered by comprehensive health insurance plans?
Coverage for oral sleep apnea devices by comprehensive health insurance plans can exhibit considerable variability, contingent upon the specific insurance provider, the individual's particular plan details, and their geographical location. In numerous regions, especially across North America, many medical insurance plans do offer partial or, in some cases, substantial or even full coverage for medically prescribed oral appliances when they are unequivocally deemed medically necessary for the treatment of formally diagnosed obstructive sleep apnea. However, it is critically imperative for patients, or their healthcare providers on their behalf, to proactively and thoroughly verify their specific insurance benefits, comprehend any associated co-pays, deductibles, or out-of-pocket maximums, and rigorously confirm all pre-authorization requirements directly with their insurance carrier prior to commencing any treatment. This diligent verification process is vital to avoid unexpected financial burdens and ensure seamless access to care.
What are the principal advantages of oral sleep apnea devices when compared to CPAP machines?
The principal advantages of oral sleep apnea devices, when juxtaposed with CPAP machines, primarily revolve around significantly enhanced patient comfort, superior convenience, and increased discretion, making them a profoundly preferred option for a substantial segment of patients. They are inherently highly portable and exceptionally travel-friendly, completely eliminating the need for bulky equipment, electrical outlets, or distilled water. Oral devices operate entirely silently, effectively addressing the noise concerns and potential sleep disturbances often associated with CPAP machines. Furthermore, they are non-invasive and aesthetically discreet, avoiding the facial straps, masks, and tubing that some CPAP users find cumbersome, claustrophobic, or visually unappealing. These combined factors collectively contribute to notably higher patient adherence rates, a superior overall treatment experience, and ultimately, improved long-term health outcomes and quality of life for appropriate candidates, offering a less intrusive pathway to managing sleep-disordered breathing.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
