PARP Inhibitor Biomarkers Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427767 | Date : Oct, 2025 | Pages : 244 | Region : Global | Publisher : MRU
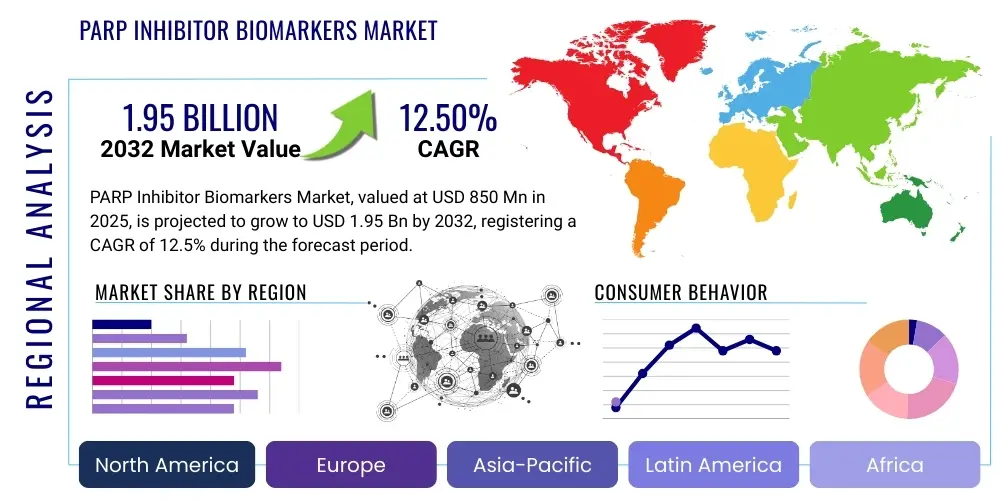
PARP Inhibitor Biomarkers Market Size
The PARP Inhibitor Biomarkers Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2025 and 2032. The market is estimated at USD 850 million in 2025 and is projected to reach USD 1.95 billion by the end of the forecast period in 2032.
PARP Inhibitor Biomarkers Market introduction
The PARP Inhibitor Biomarkers market is a critical and expanding segment within precision oncology, focusing on identifying biological indicators that predict patient response to Poly (ADP-ribose) polymerase (PARP) inhibitor therapies. These biomarkers are essential for effective patient stratification, particularly those with homologous recombination deficiency (HRD), including BRCA1/2 mutations. Their use optimizes treatment efficacy, minimizes adverse effects, and improves patient management across various cancer types.
Primary applications span ovarian, specific types of breast, metastatic castration-resistant prostate, and pancreatic cancers. In these indications, PARP inhibitors show significant clinical benefit when guided by precise biomarker identification. The development of companion diagnostics, linked to these biomarkers, is crucial for drug approval and clinical practice, ensuring appropriate patient treatment. This shift towards biomarker-driven therapy underpins the markets importance in modern oncology.
Market growth is significantly propelled by several factors. These include escalating global cancer incidence, increasing the addressable patient population. Advancements in genomic sequencing (NGS, liquid biopsy) make biomarker testing more accessible and cost-effective. A global emphasis on personalized medicine, coupled with rising R&D investments, drives innovation in biomarker discovery and validation, ensuring market expansion.
PARP Inhibitor Biomarkers Market Executive Summary
The PARP Inhibitor Biomarkers market is undergoing dynamic expansion, driven by precision medicine integration into oncology. Business trends highlight strategic partnerships between pharmaceutical and diagnostic firms for co-development of companion diagnostics, crucial for regulatory approval. There is also a trend towards consolidating diagnostic capabilities and expanding testing services to meet global demand for comprehensive genomic profiling.
Regionally, North America and Europe lead due to robust healthcare infrastructure, substantial oncology R&D, and advanced regulatory frameworks. These regions benefit from a high concentration of market players, academic centers, and strong reimbursement. Asia-Pacific is an emerging growth engine, propelled by a rapidly expanding patient pool, increasing healthcare expenditure, and improving access to advanced diagnostics. Latin America and MEA show steady growth.
Segmentation trends show BRCA1/2 mutations as the primary biomarker. However, R&D intensifies for other homologous recombination deficiency (HRD)-related markers, including genomic instability scores, expanding treatable populations. NGS and liquid biopsy adoption is rapidly increasing, offering comprehensive and less invasive testing. This evolving landscape focuses on refining diagnostic accuracy and broadening PARP inhibitor utility through deeper molecular understanding.
AI Impact Analysis on PARP Inhibitor Biomarkers Market
User inquiries about AIs influence on PARP Inhibitor Biomarkers market often focus on its potential to revolutionize biomarker discovery, refine patient selection, and enhance diagnostic accuracy. Key themes include AIs capacity to process vast genomic and clinical datasets, identify novel predictive signatures beyond traditional markers (e.g., BRCA mutations), and accelerate companion diagnostic development. Concerns involve data privacy, regulatory complexities, and the need for robust validation of AI algorithms. Overall, AI is anticipated to streamline research, personalize treatment, and improve patient outcomes by making PARP inhibitor therapy more precise and accessible.
AIs role in the PARP Inhibitor Biomarkers market extends from R&D to clinical implementation. Machine learning algorithms can sift through complex multi-omics data (genomics, transcriptomics, proteomics) to uncover subtle patterns indicating sensitivity or resistance to PARP inhibitors. This capability is critical for identifying new, actionable biomarkers not evident through conventional analysis, expanding the eligible patient population. AI also aids in developing sophisticated predictive models that integrate various clinical and molecular factors, offering nuanced understanding of individual patient responses.
- Accelerated biomarker discovery and validation via machine learning analysis of multi-omics data.
- Enhanced precision in patient stratification by integrating complex clinical and genomic profiles.
- Development of AI-powered diagnostic tools for real-time, high-throughput biomarker detection.
- Improved drug repurposing and identification of synergistic therapeutic combinations.
- Streamlined clinical trial design and patient recruitment based on refined biomarker profiles.
- Personalized treatment recommendations by predicting individual response to PARP inhibitors.
DRO & Impact Forces Of PARP Inhibitor Biomarkers Market
The PARP Inhibitor Biomarkers market is shaped by dynamic driving forces, inherent restraints, and emerging opportunities. Key drivers include escalating global cancer burden where PARP inhibitors are efficacious, coupled with a growing imperative for personalized medicine relying on accurate biomarker identification. Advancements in genomic sequencing (NGS) have made biomarker testing more accessible and comprehensive, fueling market expansion by enabling precise patient selection for targeted therapies. This push towards stratified medicine is a powerful catalyst for diagnostic innovation.
However, considerable restraints temper market growth. High costs associated with advanced genomic testing and PARP inhibitor treatments can limit patient access, especially in resource-constrained regions. Regulatory complexities and the need for stringent validation of novel biomarkers and companion diagnostics pose significant hurdles. Tumor heterogeneity and development of resistance mechanisms necessitate continuous research into new biomarkers, an expensive process. Lack of universal reimbursement policies further complicates market penetration.
Despite these challenges, substantial opportunities exist. Increasing investment in oncology R&D, particularly precision medicine, creates fertile ground for new biomarker discovery and diagnostic platform innovation. Emerging markets, with large patient populations and improving healthcare infrastructures, offer untapped potential. Liquid biopsy technologies provide less invasive and more convenient alternatives for biomarker detection, broadening patient eligibility and monitoring. Collaborative efforts between pharma, diagnostic developers, and academia drive innovation for comprehensive patient care.
Segmentation Analysis
The PARP Inhibitor Biomarkers market is meticulously segmented for a granular understanding of its components and growth opportunities. This analysis categorizes the market by biomarker type, cancer indication, end-user base, and testing technology. Each segment exhibits unique growth patterns and demand drivers, reflecting evolving precision oncology and varying clinical needs. Comprehensive analysis of these segments is crucial for strategic planning and resource allocation.
Interplay between segments is paramount. Biomarker evolution directly influences targeted indications. Advancements in testing technologies, like liquid biopsy, impact end-user adoption and application breadth. This interdependency requires stakeholders to remain agile and responsive to developments, capitalizing on opportunities and mitigating challenges effectively.
- By Biomarker Type: Primarily BRCA1/2 mutations (germline and somatic). Also HRD scores (e.g., LOH, TAI, LST), and other emerging predictive biomarkers. BRCA1/2 remains dominant, but HRD testing gains traction.
- By Indication: Encompasses ovarian cancer, specific types of breast cancer, metastatic castration-resistant prostate cancer (mCRPC), pancreatic cancer, and other solid tumors. Ovarian cancer holds significant market share due to established clinical guidelines.
- By End-User: Includes hospitals and cancer centers, independent diagnostic laboratories, academic and research institutions, and pharmaceutical/biotechnology companies. Diagnostic labs and hospital pathology departments are key segments.
- By Testing Technology: Ranging from Next-Generation Sequencing (NGS) as the gold standard, Polymerase Chain Reaction (PCR)-based assays, Immunohistochemistry (IHC), Fluorescence In Situ Hybridization (FISH), and notably, Liquid Biopsy for non-invasive detection of circulating tumor DNA (ctDNA).
PARP Inhibitor Biomarkers Market Value Chain Analysis
The value chain for the PARP Inhibitor Biomarkers market is complex, spanning from initial R&D to clinical application. Upstream, it involves fundamental research in molecular biology, genomics, and oncology to identify and validate biomarkers, requiring significant investment by academia, biotech, and pharma, often leveraging bioinformatics and AI. Raw material suppliers providing reagents, enzymes, and lab equipment are also crucial, ensuring high-quality components for diagnostic assay development.
Midstream activities focus on developing and manufacturing diagnostic assays and kits. This includes designing, optimizing, and producing platforms for specific biomarker detection (NGS panels, PCR kits, HRD assays). Diagnostic companies play a pivotal role, ensuring analytical and clinical validation for accuracy, sensitivity, and specificity. Regulatory approval (FDA, EMA) is integral, governing market introduction and clinical utility. Quality control and standardization are paramount for consistent results.
Downstream, the chain extends to distribution, utilization, and reimbursement. Distribution channels include direct sales to large institutions and indirect sales through distributors to smaller labs and clinics. Clinical laboratories and hospitals are primary end-users, integrating results into patient management. A comprehensive ecosystem of healthcare providers, oncologists, pathologists, and genetic counselors supports the process. Reimbursement policies significantly influence market access and adoption, making patient access to these critical diagnostic tools widespread.
PARP Inhibitor Biomarkers Market Potential Customers
Primary potential customers for PARP Inhibitor Biomarkers products and services are diverse, reflecting cancer care and research needs. Oncology departments in hospitals and comprehensive cancer centers are at the forefront, where clinicians rely on biomarkers to guide treatment for various cancers (ovarian, breast, prostate, pancreatic). These institutions require accurate, reliable, and timely diagnostic solutions to identify patients who will benefit most from PARP inhibitor therapies, optimizing outcomes and minimizing unnecessary drug exposure.
Diagnostic laboratories, including independent reference labs and those integrated within larger healthcare systems, represent another significant customer segment. These labs perform a wide array of molecular tests, such as genomic sequencing and HRD assays. They seek advanced, high-throughput testing platforms and validated assays offering efficiency, scalability, and adherence to stringent quality standards. Their purchasing decisions are influenced by test volume, turnaround time, cost-effectiveness, and technology robustness.
Academic and research institutions constitute a crucial customer base, particularly for novel biomarker discovery tools and advanced genomic analysis platforms. Researchers work to uncover new predictive biomarkers, understand resistance mechanisms, and explore new PARP inhibitor applications. Pharmaceutical and biotechnology companies are also key customers, integrating biomarker testing into clinical trials, developing companion diagnostics, and seeking partnerships for co-development and commercialization, requiring specialized services for trial enrollment and patient stratification.
PARP Inhibitor Biomarkers Market Key Technology Landscape
The technological landscape underpinning the PARP Inhibitor Biomarkers market is characterized by rapid innovation and integrated advanced molecular diagnostics. Next-Generation Sequencing (NGS) is a foundational technology, enabling comprehensive genomic profiling of tumor samples and germline DNA to detect BRCA1/2 mutations and assess homologous recombination deficiency (HRD) status via genomic instability scores. NGS platforms offer high throughput, analyzing multiple genes simultaneously, providing a holistic view of a patients genetic profile critical for personalized treatment decisions. Continuous NGS evolution, including accuracy, cost-efficiency, and data analysis improvements, remains a significant market driver.
Beyond NGS, other molecular technologies play vital roles. Polymerase Chain Reaction (PCR)-based assays (real-time PCR, ddPCR, multiplex PCR) are widely used for targeted BRCA mutation detection or confirming NGS findings, offering high sensitivity and speed. Immunohistochemistry (IHC) detects protein expression changes associated with HRD, though its direct utility for PARP inhibitor biomarkers is more limited than genomic methods. Fluorescence In Situ Hybridization (FISH) can detect chromosomal rearrangements indicative of HRD. Automation and integrated systems streamline testing workflows, enhancing efficiency and reducing error.
Emerging technologies, notably liquid biopsy, are significantly reshaping the market by offering less invasive alternatives to tissue biopsies. Liquid biopsy involves analyzing circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), or exosomes from blood samples to detect genetic alterations like BRCA mutations and HRD markers. This approach is valuable for patients unable to undergo tissue biopsies, for monitoring treatment response, and for detecting minimal residual disease or acquired resistance mutations. Integrating advanced bioinformatics and AI with these platforms transforms data interpretation, enabling robust analysis of complex genomic data and identifying subtle but clinically significant biomarker signatures, propelling the market towards greater precision and accessibility.
Regional Highlights
- North America: Dominates due to robust healthcare infrastructure, high R&D spending, advanced genomic testing adoption, and favorable reimbursement policies, particularly in the United States. A high concentration of key market players and academic research centers strengthens its position.
- Europe: Significant market driven by increasing cancer incidence, growing awareness of personalized medicine, and strong government support for cancer research. Germany, France, and the UK lead with advanced diagnostic capabilities and biomarker integration into national guidelines.
- Asia-Pacific: Emerging as a high-growth region due to rising healthcare expenditures, improving diagnostic infrastructure, increasing patient awareness, and a large patient pool in countries like China, Japan, and India. Economic development and greater access to advanced medical technologies are key drivers.
- Latin America: Expected to witness moderate growth, primarily driven by increasing access to advanced healthcare, improving economic conditions, and rising demand for innovative cancer therapies. Investments in healthcare infrastructure and training gradually enhance market penetration.
- Middle East & Africa: Represents a nascent but growing market, with increasing investments in healthcare infrastructure and rising prevalence of cancer contributing to gradual market expansion. International collaborations and initiatives play a crucial role in its development.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the PARP Inhibitor Biomarkers Market.- Myriad Genetics Inc.
- Foundation Medicine, Inc.
- Guardant Health, Inc.
- Invitae Corporation
- Quest Diagnostics Incorporated
- Agilent Technologies, Inc.
- QIAGEN N.V.
- Thermo Fisher Scientific Inc.
- Illumina, Inc.
- Roche Diagnostics (Genentech)
- NeoGenomics Laboratories, Inc.
- Veracyte, Inc.
Frequently Asked Questions
Analyze common user questions about the PARP Inhibitor Biomarkers market and generate a concise list of summarized FAQs reflecting key topics and concerns.What precisely are PARP Inhibitor Biomarkers and why are they important in cancer treatment?
PARP Inhibitor Biomarkers are genetic or molecular indicators, primarily BRCA1/2 mutations and homologous recombination deficiency (HRD) status, used to identify cancer patients most likely to respond to PARP inhibitor therapies. They are critical for guiding personalized treatment strategies, optimizing efficacy, and minimizing unnecessary drug exposure.
For which specific cancer types is PARP Inhibitor Biomarker testing most commonly recommended?
PARP Inhibitor Biomarker testing is most commonly recommended for ovarian cancer, specific subtypes of breast cancer, metastatic castration-resistant prostate cancer (mCRPC), and pancreatic cancer. Its utility is also being explored in other solid tumors where PARP inhibitors show potential efficacy.
What are the primary technological platforms utilized for detecting PARP Inhibitor Biomarkers?
Primary technological platforms include Next-Generation Sequencing (NGS) for comprehensive genomic profiling, Polymerase Chain Reaction (PCR)-based assays for targeted mutation detection, and increasingly, innovative liquid biopsy techniques for non-invasive analysis of circulating tumor DNA (ctDNA). These enable precise identification of relevant genetic alterations.
How is artificial intelligence (AI) influencing the evolution of the PARP Inhibitor Biomarkers market?
AI profoundly influences the market by accelerating biomarker discovery through sophisticated multi-omics data analysis, enhancing patient stratification, aiding advanced diagnostic tool development, and supporting personalized treatment recommendations for PARP inhibitor therapy, making it more targeted and effective.
What are the most significant challenges currently impacting the growth and adoption of PARP Inhibitor Biomarkers?
Significant challenges include substantial costs of advanced genomic testing and PARP inhibitor treatments, regulatory complexities for novel biomarkers, imperative for robust diagnostic assay validation, tumor heterogeneity, and the persistent issue of tumors developing acquired resistance mechanisms to PARP inhibitors.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager