Pharmaceutical Gelatin Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 431174 | Date : Nov, 2025 | Pages : 253 | Region : Global | Publisher : MRU
Pharmaceutical Gelatin Market Size
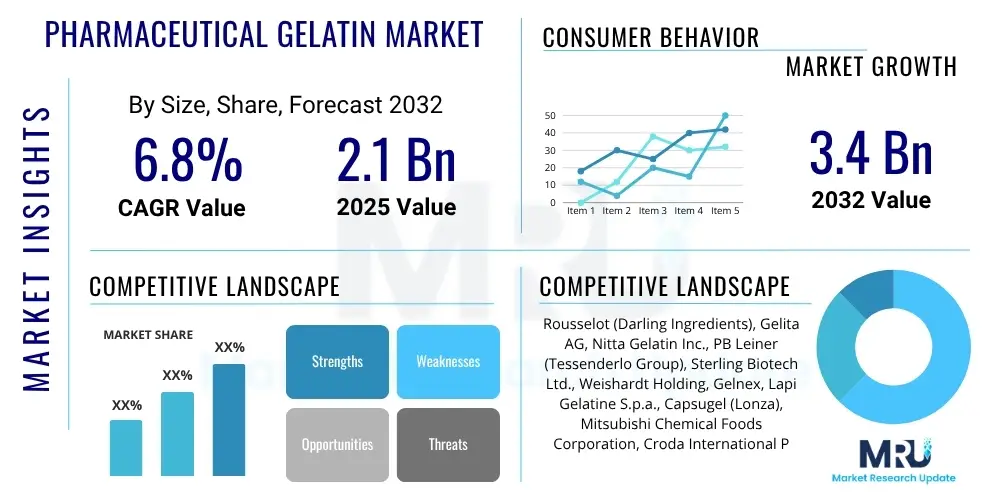
The Pharmaceutical Gelatin Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 2.1 billion in 2025 and is projected to reach USD 3.4 billion by the end of the forecast period in 2032.
Pharmaceutical Gelatin Market introduction
Pharmaceutical gelatin, a highly purified protein derived from collagen, stands as a cornerstone excipient within the global pharmaceutical industry. Its exceptional physicochemical attributes, encompassing superior gelling, film-forming, binding, and emulsifying properties, render it indispensable across a vast spectrum of drug delivery systems. This natural biopolymer, primarily sourced from bovine hides and bones, porcine skins, and increasingly, fish by-products, is meticulously processed to meet the stringent quality and safety standards required for human consumption in medicinal applications. Its versatility allows for its integration into various dosage forms, ensuring both therapeutic efficacy and patient convenience.
The product's principal applications span the encapsulation of active pharmaceutical ingredients (APIs) in hard and soft capsules, where it provides robust protection against degradation while facilitating controlled drug release. Beyond encapsulation, gelatin serves as an effective binder in tablet formulations, ensuring structural integrity and consistent dissolution profiles. It is also utilized in coatings, suppositories, and as a stabilizing agent in vaccines, plasma expanders, and other parenteral preparations. The inherent benefits of pharmaceutical gelatin, such as its biocompatibility, biodegradability, non-allergenic nature, and established safety record, significantly contribute to its widespread acceptance and continued demand in sophisticated drug formulations worldwide.
Driving the sustained growth of this market are several key factors, including the continuous expansion of the global pharmaceutical sector, a rising geriatric population necessitating increased medication intake, and the escalating prevalence of chronic diseases. Innovations in drug delivery technologies and the growing consumer preference for convenient oral dosage forms, particularly softgels for vitamins, dietary supplements, and specialized therapies, further amplify market demand. As the industry seeks natural, effective, and safe excipient solutions, pharmaceutical gelatin remains a critical component, underpinning the development of modern medicines and contributing to improved public health outcomes.
Pharmaceutical Gelatin Market Executive Summary
The Pharmaceutical Gelatin Market is experiencing dynamic growth, primarily fueled by the burgeoning global pharmaceutical industry and the escalating demand for advanced drug delivery systems. Key business trends indicate a strategic emphasis on diversifying raw material sourcing to include more fish-derived options, driven by religious and dietary considerations, alongside continuous investment in research and development to enhance gelatin's functional properties. Consolidation activities among major manufacturers and strategic partnerships with pharmaceutical companies are also shaping the competitive landscape, aimed at expanding market share and optimizing supply chain efficiencies. The drive towards sustainable and ethical sourcing practices is increasingly influencing operational strategies across the industry, reflecting broader corporate social responsibility initiatives.
From a regional perspective, the Asia Pacific region is poised for significant expansion, attributed to its rapidly growing pharmaceutical manufacturing base, increasing healthcare expenditure, and a substantial patient demographic in countries like China and India. North America and Europe, as mature markets, continue to demonstrate stable demand, characterized by stringent regulatory environments, sophisticated research infrastructure, and the presence of numerous global pharmaceutical giants. Emerging economies in Latin America and the Middle East and Africa are also showing promising growth trajectories, supported by improving healthcare accessibility and rising investments in pharmaceutical production capabilities.
Segmentation trends highlight the enduring dominance of bovine and porcine gelatin due to their widespread availability and cost-effectiveness, although fish-derived gelatin is registering a notable surge in demand for its hypoallergenic properties and suitability for specific dietary requirements. Hard capsules and softgels remain the most significant application segments, benefiting from their ease of administration and effectiveness in protecting active pharmaceutical ingredients. The market also observes steady growth in gelatin's utilization within tablet binding, coatings, and specialized biologics, underscoring its indispensable role as a versatile excipient across a broad spectrum of pharmaceutical end-uses.
AI Impact Analysis on Pharmaceutical Gelatin Market
User inquiries concerning AI's influence on the Pharmaceutical Gelatin Market frequently center on how artificial intelligence can revolutionize various facets of production and application. A primary area of interest involves AI's potential to optimize manufacturing efficiency, enhance quality control processes, and significantly streamline complex supply chain logistics for gelatin. Users are keen to understand the predictive capabilities of AI in anticipating market demand, improving raw material sourcing strategies, and accelerating the development of novel gelatin-based formulations with improved functional attributes. The dialogue often includes concerns regarding the substantial initial investment costs associated with AI integration, the necessity for specialized technical expertise to implement and manage AI systems, and the potential impact on traditional workforce roles as automation advances. Overall expectations center on AI driving significant advancements in operational efficiency, waste reduction, and the expedited pace of research and development, ultimately leading to more cost-effective and higher-quality pharmaceutical products that meet evolving industry standards.
- Enhanced quality control through AI-powered image analysis and predictive analytics for raw material assessment and final product consistency, minimizing defects and ensuring adherence to pharmacopeial standards.
- Optimized manufacturing processes by leveraging machine learning algorithms to fine-tune extraction parameters, control drying conditions, reduce energy consumption, and minimize waste generation.
- Predictive demand forecasting and sophisticated supply chain management for gelatin and its raw materials, improving inventory management, reducing lead times, and mitigating supply disruptions.
- Accelerated research and development of novel gelatin formulations, including advanced drug encapsulation techniques and modified release systems, by simulating molecular interactions and predicting performance characteristics.
- Automation of routine tasks in laboratories and production lines, leading to greater precision, efficiency, and safety in gelatin processing and quality assurance.
- Support for personalized medicine development, where AI can help tailor gelatin-based drug delivery systems for specific patient needs based on predicted efficacy and absorption profiles.
- Improved traceability and regulatory compliance across the entire value chain through AI-driven data management and auditing systems, ensuring transparency from raw material sourcing to final product distribution.
DRO & Impact Forces Of Pharmaceutical Gelatin Market
The Pharmaceutical Gelatin Market is significantly propelled by several robust drivers. Firstly, the relentless expansion of the global pharmaceutical industry, fueled by an aging population, increasing prevalence of chronic diseases, and rising healthcare expenditure, inherently escalates the demand for high-quality excipients. Gelatin's natural origin, coupled with its excellent biocompatibility, biodegradability, and non-allergenic properties, positions it as an ideal choice for pharmaceutical formulations, especially in oral solid dosage forms. The growing popularity of convenient dosage forms such as hard capsules and softgels, for both prescription drugs and over-the-counter supplements, further amplifies its consumption. Additionally, its utility as a stabilizer in vaccines and blood plasma expanders underscores its critical role in various therapeutic areas, contributing to sustained market growth.
Despite these strong drivers, the market faces notable restraints. The increasing availability and adoption of alternative excipients, including synthetic polymers like HPMC (hydroxypropyl methylcellulose) and pullulan, pose a significant challenge. These alternatives are often favored due to dietary, religious, and ethical concerns associated with animal-derived products, particularly in specific consumer demographics. Stringent regulatory scrutiny and the complex, time-consuming approval processes for pharmaceutical-grade excipients also present barriers, increasing compliance costs for manufacturers. Furthermore, the inherent volatility in raw material prices—animal hides, bones, and fish skins—can lead to unpredictable production costs and impact the overall profitability and stability of the market.
Opportunities within the Pharmaceutical Gelatin Market are substantial and multifaceted. Emerging economies, particularly in the Asia Pacific and Latin American regions, offer lucrative growth prospects due to their burgeoning pharmaceutical sectors, improving healthcare infrastructure, and increasing access to modern medicines. There is also significant potential in the development of advanced gelatin formulations tailored for novel drug delivery systems, such as controlled-release matrices, targeted therapies, and specialized parenteral applications. Continuous innovation in processing technologies aimed at enhancing gelatin's functional properties, alongside a growing emphasis on sustainable and ethically sourced gelatin, presents avenues for market differentiation and expansion. The market's competitive dynamics are shaped by the bargaining power of a relatively concentrated group of raw material suppliers and a fragmented base of pharmaceutical buyers, the persistent threat of substitute excipients, and the high entry barriers stemming from capital intensity, technological know-how, and strict regulatory requirements, leading to intense rivalry among established gelatin manufacturers.
Segmentation Analysis
The Pharmaceutical Gelatin Market is meticulously segmented to provide a comprehensive understanding of its intricate structure, diverse applications, and underlying market dynamics. This detailed breakdown allows for a granular analysis of various market components, facilitating insights into consumer preferences, technological advancements, and regional disparities. Understanding these segmentations is crucial for industry stakeholders, including manufacturers, suppliers, and pharmaceutical companies, to identify pivotal market trends, strategically position their products, and tailor offerings to meet the specific demands of a rapidly evolving global healthcare landscape. Each segment contributes uniquely to the overall market valuation and growth trajectory.
- By Source
- Bovine: Predominantly from bovine hides and bones, known for high strength and widespread availability.
- Porcine: Derived from pig skin, offering specific gelling properties and cost-effectiveness.
- Fish: Sourced from fish skins, appealing to dietary restrictions and exhibiting unique thermal properties.
- Other Animal Sources: Includes poultry and other less common animal by-products, explored for niche applications.
- By Type
- Type A (Acid-treated): Produced from acid-processed raw materials, typically porcine and fish, resulting in an isoelectric point between pH 7 and pH 9.
- Type B (Alkali-treated): Derived from alkali-processed raw materials, primarily bovine, with an isoelectric point between pH 4.7 and pH 5.2.
- By Application
- Hard Capsules: Dominant use for encapsulating solid and powdered active pharmaceutical ingredients.
- Softgels: Widely used for liquid, semi-solid, and oil-based formulations, offering excellent bioavailability.
- Tablets (Binders, Coatings): Functions as a binder for tablet cohesion and as a coating for protection and controlled release.
- Biologics (Vaccines, Plasma Expanders): Utilized as a stabilizer in vaccines and as a blood volume expander.
- Suppositories: Provides a thermoreversible base for rectal or vaginal drug delivery.
- Wound Dressings: Used in biocompatible wound care applications for healing and protection.
- Other Pharmaceutical Applications: Includes emulsions, microencapsulation, and medical device coatings.
- By End-use
- Pharmaceutical Manufacturers: Largest consumers for producing a wide range of medications.
- Contract Manufacturing Organizations (CMOs): Companies outsourcing drug development and manufacturing, requiring diverse gelatin types.
- Research & Academic Institutes: Utilizing gelatin for drug discovery, formulation research, and biomaterial studies.
- Nutraceutical Companies: Employing gelatin primarily for encapsulating vitamins, minerals, and dietary supplements.
Value Chain Analysis For Pharmaceutical Gelatin Market
The value chain for the Pharmaceutical Gelatin Market commences with critical upstream activities centered on the responsible sourcing and initial processing of raw materials. This foundational stage involves a global network of suppliers providing animal by-products, predominantly bovine hides and bones, porcine skins, and an increasing volume of fish skins. Emphasis is placed on ethical sourcing, animal welfare, and rigorous quality inspection of these raw materials to ensure they meet preliminary standards for collagen extraction. Initial processing steps typically include cleaning, degreasing, and grinding, which prepare the collagen-rich tissues for subsequent chemical treatments, directly impacting the quality and yield of the final gelatin product. Strategic partnerships with raw material providers are crucial for ensuring a consistent, high-quality, and traceable supply.
Midstream in the value chain, specialized gelatin manufacturers undertake complex conversion processes. This stage involves the precise chemical treatment of collagen (either acid treatment for Type A gelatin or alkaline treatment for Type B gelatin), followed by hot water extraction, which solubilizes the collagen into gelatin. Subsequent purification steps, including filtration, demineralization, and concentration, are critical for removing impurities and achieving pharmaceutical-grade purity. Finally, the gelatin solution is dried (e.g., via spray drying or freeze-drying) and milled into a consistent powder or granule form. This manufacturing phase requires significant capital investment in advanced processing technologies, adherence to Good Manufacturing Practices (GMP), and stringent quality control measures to ensure the gelatin meets all pharmacopeial specifications for molecular weight distribution, gelling strength, viscosity, and microbial purity.
Downstream activities focus on the distribution and ultimate utilization of pharmaceutical gelatin by various end-users. The primary consumers are large-scale pharmaceutical manufacturers and Contract Manufacturing Organizations (CMOs) that integrate gelatin into their diverse drug formulations. Distribution channels can be direct, involving bulk sales and long-term contracts between gelatin producers and major pharmaceutical players, or indirect, facilitated by specialized distributors and agents who cater to smaller manufacturers, nutraceutical companies, and research institutions. The efficiency of this distribution network, including cold chain management and robust logistics, is essential for maintaining product integrity and ensuring timely delivery across global markets. Effective post-sales support and technical assistance further enhance customer satisfaction and solidify long-term relationships within this critical segment of the pharmaceutical supply chain.
Pharmaceutical Gelatin Market Potential Customers
The primary potential customers for pharmaceutical gelatin are robust entities within the global healthcare and nutraceutical industries that demand high-quality, biocompatible, and safe excipients for the formulation and delivery of a wide array of therapeutic and supplemental products. Foremost among these are large-scale pharmaceutical manufacturers, which represent the largest consumption segment. These companies leverage pharmaceutical gelatin extensively for producing prescription and over-the-counter medications, particularly those requiring hard capsules, softgels, coated tablets, and as stabilizers in more complex biological formulations like vaccines and plasma expanders. Their purchasing decisions are driven by stringent regulatory compliance, product consistency, supplier reliability, and the ability to source gelatin in bulk quantities that meet specific pharmacopeial standards.
Another significant customer segment comprises Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs). As pharmaceutical companies increasingly outsource various stages of drug development and manufacturing, CMOs/CDMOs require a diverse portfolio of pharmaceutical-grade gelatin to fulfill varied client requirements across different drug forms and therapeutic areas. Their demand is often characterized by a need for flexibility in gelatin types and specifications, coupled with competitive pricing and technical support. These organizations act as crucial intermediaries, converting raw gelatin into finished pharmaceutical products for a multitude of global clients, making them pivotal buyers in the market. Their growth directly correlates with the outsourcing trends within the broader pharmaceutical industry.
Beyond these major players, nutraceutical companies constitute a substantial and rapidly expanding customer base. These firms predominantly utilize gelatin for encapsulating vitamins, minerals, dietary supplements, and functional food ingredients, primarily in softgel formats due to their ease of swallowing and effective protection of active ingredients. Additionally, research and academic institutions, alongside specialized biotechnology firms, are also important, albeit smaller, customers. They procure pharmaceutical gelatin for experimental drug delivery systems, tissue engineering applications, biomaterial research, and the development of advanced medical devices. The customer acquisition strategy for gelatin suppliers must therefore be multi-faceted, addressing the specific technical, regulatory, and commercial needs of each distinct end-user category.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 2.1 billion |
| Market Forecast in 2032 | USD 3.4 billion |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Rousselot (Darling Ingredients), Gelita AG, Nitta Gelatin Inc., PB Leiner (Tessenderlo Group), Sterling Biotech Ltd., Weishardt Holding, Gelnex, Lapi Gelatine S.p.a., Capsugel (Lonza), Mitsubishi Chemical Foods Corporation, Croda International Plc, Tessenderlo Group, Junca Gelatines, Ewald-Gelatine GmbH, Roxlor LLC, Suheung Co. Ltd., Neocell Corporation, Advanced BioNutrition Corp., Hainan Qiaofeng Chemical Co., Ltd., Dongbao Bio-Tech Co., Ltd. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Pharmaceutical Gelatin Market Key Technology Landscape
The technological landscape of the Pharmaceutical Gelatin Market is characterized by continuous innovation aimed at optimizing extraction processes, enhancing product purity, and expanding functional applications. Traditional manufacturing relies on precise acid or alkaline hydrolysis of collagen, followed by hot water extraction. However, contemporary advancements focus on more sophisticated methodologies. These include enzyme-assisted extraction techniques, which offer milder processing conditions, reduced chemical usage, and the potential to yield gelatin with more specific molecular weight distributions and tailored physiochemical properties. Such innovations are crucial for developing customized gelatin variants suitable for increasingly complex drug delivery systems and biopharmaceutical formulations, ensuring higher efficacy and safety profiles.
Furthermore, significant technological efforts are dedicated to purification and stringent quality control. State-of-the-art purification techniques, such as ultrafiltration, nanofiltration, and ion exchange chromatography, are employed to effectively remove impurities, control microbial load, and ensure heavy metal content is well below regulatory limits. These processes are vital for achieving pharmaceutical-grade gelatin that complies with global pharmacopeial standards, including USP, EP, and JP. Advanced analytical methodologies, including high-performance liquid chromatography (HPLC), gel permeation chromatography (GPC), Fourier-transform infrared (FTIR) spectroscopy, and rheological measurements, are routinely utilized to characterize gelatin batches, verifying consistency in gelling strength, viscosity, and molecular integrity, which are critical for predictable performance in pharmaceutical applications.
Beyond basic production, the key technology landscape also encompasses sophisticated drug encapsulation and delivery technologies that leverage gelatin's unique properties. This includes innovations in hard and soft capsule manufacturing, such as seamless encapsulation, liquid-filled hard capsules, and enteric-coated capsules, which extend the range of drugs that can be effectively delivered. Research into novel cross-linking agents and the blending of gelatin with other biocompatible polymers is also prevalent, aimed at modifying release profiles, enhancing stability, and developing targeted drug delivery systems. Moreover, the industry is increasingly investing in sustainable processing technologies, including resource-efficient operations and waste valorization techniques, to reduce the environmental footprint of gelatin production, aligning with global efforts towards a greener pharmaceutical supply chain and improving overall operational efficiency.
Regional Highlights
- North America: A highly mature and dominant market, characterized by a robust pharmaceutical industry, substantial investments in R&D, and the widespread adoption of advanced drug delivery systems. Stringent regulatory frameworks, such as those from the FDA, ensure high-quality standards for pharmaceutical gelatin, driving consistent demand for premium-grade products and fostering innovation.
- Europe: Represents another established and significant market, boasting strong pharmaceutical manufacturing capabilities, a high focus on innovation, and rigorous regulatory oversight by bodies like the European Medicines Agency (EMA). This region is a key hub for pharmaceutical research and development, particularly in biologics and advanced therapies, which sustains a high demand for high-grade gelatin.
- Asia Pacific (APAC): Positioned as the fastest-growing regional market, propelled by rapid economic development, increasing healthcare expenditure, a vast and aging patient population, and the burgeoning expansion of generic drug manufacturing hubs in countries like China, India, and Japan. The rising demand for affordable medicines and nutraceuticals significantly boosts gelatin consumption in this dynamic region.
- Latin America: An emerging market exhibiting steady and promising growth due to improving healthcare infrastructure, increasing access to modern medicines, and a rising awareness of health and wellness among its population. Countries such as Brazil, Mexico, and Argentina are key contributors to the regional demand for pharmaceutical gelatin as their domestic pharmaceutical industries expand.
- Middle East and Africa (MEA): A nascent but rapidly developing market, driven by significant governmental initiatives to enhance healthcare services, increasing foreign direct investment in the pharmaceutical sector, and a growing demand for high-quality drug formulations. Specific religious and dietary preferences in certain parts of the MEA region also contribute to a rising demand for alternative gelatin types, particularly fish gelatin.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Pharmaceutical Gelatin Market.- Rousselot (Darling Ingredients)
- Gelita AG
- Nitta Gelatin Inc.
- PB Leiner (Tessenderlo Group)
- Sterling Biotech Ltd.
- Weishardt Holding
- Gelnex
- Lapi Gelatine S.p.a.
- Capsugel (Lonza)
- Mitsubishi Chemical Foods Corporation
- Croda International Plc
- Tessenderlo Group
- Junca Gelatines
- Ewald-Gelatine GmbH
- Roxlor LLC
- Suheung Co. Ltd.
- Neocell Corporation
- Advanced BioNutrition Corp.
- Hainan Qiaofeng Chemical Co., Ltd.
- Dongbao Bio-Tech Co., Ltd.
Frequently Asked Questions
What is pharmaceutical gelatin and its primary uses?
Pharmaceutical gelatin is a high-purity protein derived from animal collagen, serving as a vital excipient in the pharmaceutical industry. Its primary uses include encapsulating active ingredients in hard and soft capsules, functioning as a binder in tablets, and stabilizing sensitive formulations like vaccines and plasma expanders, leveraging its biocompatibility and gelling properties.
What are the key raw material sources for pharmaceutical gelatin production?
The main raw material sources for pharmaceutical gelatin production are bovine hides and bones, porcine skins, and fish skins. Bovine and porcine sources are widely utilized, while fish gelatin is gaining increasing traction, especially in regions with specific dietary, religious, or technical requirements, offering versatility in sourcing options.
How do Type A and Type B gelatins differ?
Type A gelatin is produced through an acid treatment process, typically from porcine or fish collagen, resulting in an isoelectric point between pH 7 and pH 9. Type B gelatin is derived from an alkaline treatment, usually from bovine collagen, with an isoelectric point between pH 4.7 and pH 5.2. These differences in processing and isoelectric points impact their functional properties and suitability for various pharmaceutical applications.
What are the major applications driving the Pharmaceutical Gelatin Market?
The major applications driving market growth include hard capsules for solid dose forms, softgels for liquid and semi-solid medications, and its use in tablet binding and coatings. Additionally, its role as a stabilizer in biologics such as vaccines and blood volume expanders, along with applications in suppositories and wound dressings, significantly contributes to demand.
What challenges does the Pharmaceutical Gelatin Market face from alternatives?
The market faces significant challenges from the growing availability and adoption of alternative excipients, such as plant-based polymers like HPMC and pullulan. These alternatives are increasingly preferred due to rising ethical, religious, and dietary concerns associated with animal-derived products, alongside potential cost efficiencies and specific formulation advantages they may offer.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
