Pregnancy Urine Rapid Diagnostic Test Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427692 | Date : Oct, 2025 | Pages : 243 | Region : Global | Publisher : MRU
Pregnancy Urine Rapid Diagnostic Test Market Size
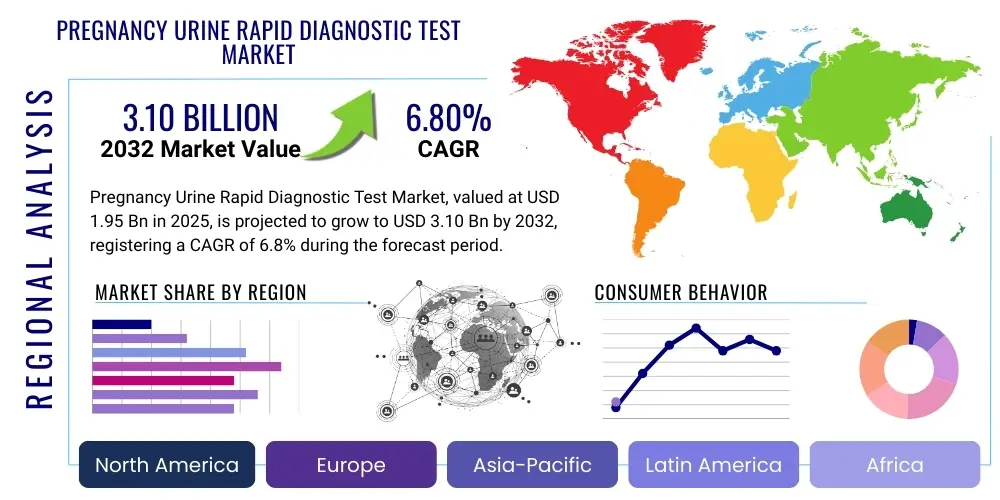
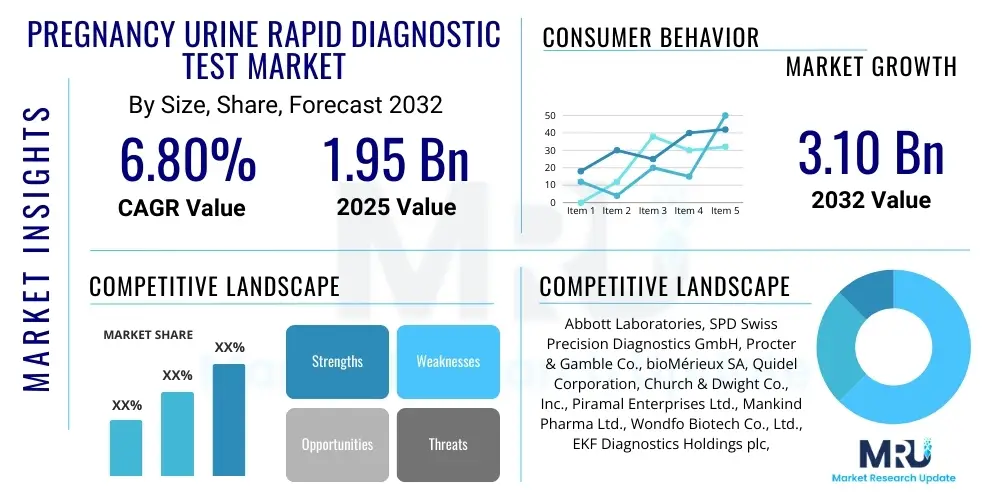
The Pregnancy Urine Rapid Diagnostic Test Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 1.95 Billion in 2025 and is projected to reach USD 3.10 Billion by the end of the forecast period in 2032. This growth is primarily driven by increasing awareness regarding early pregnancy detection, the convenience offered by home-based testing solutions, and a rising number of unplanned pregnancies globally. The affordability and accessibility of these tests further contribute to their widespread adoption across various demographics.
The market expansion is also supported by continuous advancements in test accuracy and sensitivity, alongside a growing emphasis on reproductive health and family planning initiatives worldwide. Manufacturers are focusing on developing user-friendly designs and incorporating digital features, which enhance the consumer experience and expand the market reach, especially in technologically forward regions. The increasing penetration of e-commerce platforms also facilitates easier access to these diagnostic tools, propelling market volume and value.
Pregnancy Urine Rapid Diagnostic Test Market introduction
The Pregnancy Urine Rapid Diagnostic Test Market encompasses a range of products designed for the early and convenient detection of pregnancy through the analysis of human chorionic gonadotropin (hCG) hormone levels in urine. These tests are primarily utilized to determine if a woman is pregnant, often offering results within minutes of application. The core products include strip tests, cassette tests, and midstream tests, each varying in design but operating on the principle of lateral flow immunoassay. They leverage antibodies that specifically bind to hCG, producing a visual indicator, typically a line or a digital display, to signify pregnancy.
Major applications for these tests span across home-based personal use, clinical settings for initial screening, and diagnostic laboratories. The primary benefit lies in their user-friendliness, speed, and high level of privacy, allowing individuals to ascertain their pregnancy status quickly and discreetly. This immediate feedback enables timely medical consultations and informed decision-making regarding reproductive health. Key driving factors propelling market growth include the global increase in birth rates, a rise in unplanned pregnancies, heightened awareness regarding early prenatal care, and the overall convenience and affordability that these rapid diagnostic tools provide.
Pregnancy Urine Rapid Diagnostic Test Market Executive Summary
The Pregnancy Urine Rapid Diagnostic Test Market is experiencing robust growth, driven by key business trends such as technological advancements leading to more accurate and sensitive tests, increased investment in research and development for digital and smart test kits, and the expansion of e-commerce channels facilitating greater product accessibility. Companies are focusing on product differentiation through enhanced user experience and integration with mobile health applications. Regional trends indicate strong growth in Asia-Pacific due to large populations, increasing disposable incomes, and improving healthcare infrastructure, while North America and Europe maintain significant market shares owing to high awareness and advanced healthcare systems. Emerging markets in Latin America and Africa are also showing promising expansion.
Segmentation trends highlight a growing preference for digital pregnancy tests due to their clear results and advanced features, though traditional strip and midstream tests continue to dominate in terms of volume due to their affordability. The home care settings segment remains the largest end-user, while online pharmacies are rapidly becoming a preferred distribution channel, offering convenience and privacy. The market is characterized by a mix of established global players and emerging regional manufacturers, leading to competitive pricing and continuous innovation. Overall, the markets trajectory is positive, supported by consumer demand for reliable, accessible, and user-friendly pregnancy detection solutions.
AI Impact Analysis on Pregnancy Urine Rapid Diagnostic Test Market
User inquiries regarding AIs impact on the Pregnancy Urine Rapid Diagnostic Test Market often revolve around enhanced accuracy, predictive capabilities, integration with broader digital health platforms, and the potential for personalized insights beyond simple positive/negative results. Consumers are curious about how AI can minimize false readings, provide more nuanced information about gestational age, and offer actionable advice based on test results in conjunction with other health data. Theres also a significant interest in data privacy and the ethical implications of collecting and processing sensitive reproductive health information through AI-powered devices or applications. Expectations include more sophisticated diagnostic support, better integration into family planning strategies, and the evolution of at-home testing from a standalone product to a connected health tool.
The convergence of AI with rapid diagnostic tests holds the promise of transforming current practices by offering a layer of intelligent analysis and personalized guidance. While the core chemical reaction of these tests remains biochemical, AI can significantly augment the interpretation, tracking, and subsequent user experience. This includes leveraging machine learning algorithms to analyze subtle color changes or digital signals more precisely than the human eye, reducing the margin for user error, and providing a clearer, more definitive result. Furthermore, AI can facilitate the integration of test outcomes with personal health records and fertility tracking applications, offering a holistic view of reproductive health and enabling proactive health management.
- Improved diagnostic accuracy through advanced image analysis and pattern recognition, minimizing false positives and negatives.
- Integration with mobile health applications to track results, predict fertile windows, and offer personalized health insights.
- Enhanced user experience with AI-powered instructions and troubleshooting, reducing user error during test administration.
- Potential for predictive analytics in fertility management by correlating test results with other physiological data points.
- Development of smart tests that communicate results directly to healthcare providers, streamlining early prenatal care pathways.
- Personalized recommendations for follow-up actions or lifestyle adjustments based on comprehensive data analysis.
DRO & Impact Forces Of Pregnancy Urine Rapid Diagnostic Test Market
The Pregnancy Urine Rapid Diagnostic Test Market is propelled by several key drivers, including the growing global population, an increase in average maternal age, and the associated rise in unplanned pregnancies, necessitating early detection for better health outcomes. Enhanced awareness campaigns by public health organizations and widespread product availability across diverse retail and online channels significantly contribute to market expansion. Restraints on the market primarily include concerns over test accuracy, particularly the potential for false negatives in early stages of pregnancy or false positives due to certain medical conditions, which often necessitate confirmation through clinical laboratory tests. Additionally, regulatory complexities and the varying standards across different regions can pose challenges for manufacturers aiming for global market penetration. The environmental impact of single-use plastic test kits also presents a growing concern for sustainable market development.
Opportunities within this market are substantial, driven by continuous innovation in test sensitivity and specificity, the development of multi-parameter tests that offer more comprehensive health insights, and the integration of digital features for enhanced user experience and data tracking. Expanding into underserved emerging economies with affordable and accessible testing solutions represents a significant growth avenue. Impact forces shaping the market include rapid technological advancements leading to more sophisticated and user-friendly products, evolving regulatory frameworks that can either facilitate or constrain market entry and product innovation, and shifts in consumer preferences towards convenience, privacy, and digital health solutions. Economic conditions, including disposable income levels and healthcare spending, also play a crucial role in influencing product adoption and market demand across different regions.
Segmentation Analysis
The Pregnancy Urine Rapid Diagnostic Test market is meticulously segmented to provide a granular understanding of its diverse components and growth dynamics. These segmentations are crucial for identifying specific market trends, consumer preferences, and strategic opportunities for manufacturers and distributors. The market can be categorized by product type, reflecting the different formats and technologies of tests available; by test type, indicating the specific hCG measurement methods; by end-user, illustrating who the primary consumers are; and by distribution channel, detailing how these products reach the consumer base. This comprehensive breakdown allows for targeted marketing strategies and product development initiatives, ensuring that the needs of various consumer groups are effectively met across different geographical and economic landscapes.
- By Product Type
- Strip Tests
- Cassette Tests
- Midstream Tests
- Digital Tests
- By Test Type
- HCG Qualitative Tests
- HCG Quantitative Tests (though less common in rapid urine tests, some advanced versions or integrated systems may offer a semi-quantitative indication)
- By End-User
- Home Care Settings
- Hospitals & Clinics
- Diagnostic Laboratories
- By Distribution Channel
- Pharmacies & Drug Stores
- Online Pharmacies
- Supermarkets & Hypermarkets
- Direct Sales (to healthcare institutions)
Pregnancy Urine Rapid Diagnostic Test Market Value Chain Analysis
The value chain for the Pregnancy Urine Rapid Diagnostic Test Market begins with upstream activities involving the sourcing and manufacturing of essential raw materials and components. This includes the production of monoclonal antibodies specific to hCG, various chemical reagents, nitrocellulose membranes, plastic casings, and packaging materials. Key suppliers in this segment focus on quality, cost-effectiveness, and consistent supply, as these are critical for the reliability and affordability of the final product. Research and development plays a significant upstream role, constantly working on improving test sensitivity, specificity, and shelf life, as well as integrating new technologies like digital interfaces. Manufacturers then assemble these components, ensuring rigorous quality control and adherence to regulatory standards during the production process.
Downstream activities involve the distribution, marketing, and sales of the finished pregnancy tests to end-users. The distribution channel is multifaceted, encompassing both direct and indirect routes. Direct sales are typically to large institutional buyers such as hospitals, clinics, and government health programs, where bulk procurement is common. Indirect channels involve a broader network of distributors, wholesalers, and retailers, including pharmacies, drug stores, supermarkets, hypermarkets, and increasingly, online pharmacies and e-commerce platforms. Marketing and promotional activities are crucial at this stage to build brand awareness, educate consumers, and highlight product benefits. Effective logistics and supply chain management are essential to ensure timely delivery and widespread availability, ultimately influencing market penetration and consumer access.
Pregnancy Urine Rapid Diagnostic Test Market Potential Customers
The primary potential customers for Pregnancy Urine Rapid Diagnostic Tests are women of childbearing age, particularly those who are sexually active and suspect pregnancy, or those actively trying to conceive and seeking early confirmation. This demographic segment values convenience, privacy, and rapid results, making home-based testing a preferred first step. Beyond individual consumers, healthcare providers such as gynecologists, obstetricians, and general practitioners represent a significant customer segment, utilizing these tests for initial screening in clinical settings before confirming with blood tests or ultrasound. Fertility clinics also frequently employ these tests as part of their patient management protocols.
Additionally, family planning centers, public health initiatives, and non-governmental organizations involved in reproductive health outreach programs are key institutional buyers, often distributing tests to underserved populations to promote early prenatal care and informed reproductive choices. Pharmacies, drug stores, and major retail chains are also direct customers from the manufacturers perspective, as they serve as the primary retail outlets for the end-users. The customer base is broad and diverse, unified by the common need for accurate, accessible, and timely pregnancy detection. This wide spectrum of end-users and buyers underscores the markets robust demand and the importance of tailored product offerings and distribution strategies.
Pregnancy Urine Rapid Diagnostic Test Market Key Technology Landscape
The core technology underpinning the Pregnancy Urine Rapid Diagnostic Test market is the lateral flow immunoassay (LFIA). This technology utilizes a nitrocellulose membrane impregnated with specific antibodies to detect the presence of human chorionic gonadotropin (hCG) in urine. Monoclonal antibodies, highly specific to different epitopes of the hCG molecule, are crucial components that ensure high sensitivity and specificity, enabling detection of pregnancy even at very low hormone concentrations in early stages. Advancements in this area focus on developing more robust and sensitive antibody pairs to improve detection limits and reduce false results.
Beyond the basic LFIA, the market is increasingly integrating digital display technology and microelectronics. Digital pregnancy tests eliminate the ambiguity of interpreting faint lines, providing clear "Pregnant" or "Not Pregnant" results, sometimes even estimating gestational age. This involves optical sensors that read the test lines and microprocessors to interpret the signal and display it digitally. Further technological evolution includes smartphone app integration, where users can scan their test results, track cycles, receive personalized health information, and potentially connect with healthcare providers. This move towards smart diagnostics enhances user experience, provides additional functionalities, and aligns with the broader trend of connected health. Material science improvements in membrane technology and reagent stability also contribute significantly to extending shelf life and improving test performance.
Regional Highlights
- North America: A mature market characterized by high consumer awareness, advanced healthcare infrastructure, and significant disposable income. The presence of key market players and a strong emphasis on digital health solutions contribute to sustained growth. Focus on product innovation and premium offerings.
- Europe: Exhibits steady growth driven by a well-established healthcare system, increasing birth rates in some regions, and favorable reimbursement policies. Strict regulatory standards ensure high product quality and safety. Germany, UK, and France are key contributors.
- Asia Pacific: The fastest-growing region, fueled by large population bases, rising disposable incomes, improving healthcare access, and increasing awareness of reproductive health. Emerging economies like China and India present vast untapped potential. Focus on affordability and accessibility.
- Latin America: Showing promising growth due to increasing healthcare spending, growing awareness, and economic development. Brazil and Mexico are leading countries, with rising demand for convenient and reliable diagnostic tools.
- Middle East & Africa: An emerging market with significant growth potential, primarily driven by improving healthcare infrastructure, increasing government initiatives for womens health, and a growing population. Challenges include varying regulatory landscapes and limited access in some remote areas.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Pregnancy Urine Rapid Diagnostic Test Market.- Abbott Laboratories
- SPD Swiss Precision Diagnostics GmbH (Clearblue)
- Procter & Gamble Co.
- bioMérieux SA
- Quidel Corporation
- Church & Dwight Co., Inc. (FIRST RESPONSE)
- Piramal Enterprises Ltd. (i-can)
- Mankind Pharma Ltd. (Prega News)
- Wondfo Biotech Co., Ltd.
- EKF Diagnostics Holdings plc
- Hologic, Inc.
- Cardinal Health Inc.
- AccuQuik Diagnostics
- RunBio Biotech Co., Ltd.
- Easy Healthcare Corporation (Premom)
Frequently Asked Questions
How accurate are home pregnancy tests?
Home pregnancy tests are highly accurate, typically over 99% reliable when used correctly and at the appropriate time, usually after a missed period. However, accuracy can vary depending on factors like test sensitivity, user technique, and the timing of the test relative to ovulation and implantation.
When is the best time to take a rapid pregnancy test?
For the most reliable results, it is generally recommended to take a rapid pregnancy test on the first day of your missed period or later. Testing with your first morning urine is often advised as it contains the highest concentration of hCG hormone, leading to clearer results.
Can certain medications or conditions affect pregnancy test results?
Yes, certain medications containing hCG, such as fertility drugs, can lead to false positive results. Rare medical conditions like certain types of ovarian cysts or ectopic pregnancies can also elevate hCG levels, potentially causing a positive test without a viable intrauterine pregnancy. Always consult a healthcare professional for definitive diagnosis.
Are digital pregnancy tests more reliable than traditional line tests?
Digital pregnancy tests generally offer the same level of accuracy as traditional line tests, as both rely on detecting hCG. However, digital tests provide a clear "Pregnant" or "Not Pregnant" result, eliminating the ambiguity of interpreting faint lines, which many users find more reassuring and easier to read.
Where can I purchase pregnancy urine rapid diagnostic tests?
Pregnancy urine rapid diagnostic tests are widely available for purchase at various retail locations including pharmacies, drug stores, supermarkets, hypermarkets, and online pharmacies. They can also be found on major e-commerce platforms, offering convenience and discreet delivery options to consumers.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager