Primary Biliary Cholangitis Therapeutics Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429696 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Primary Biliary Cholangitis Therapeutics Market Size
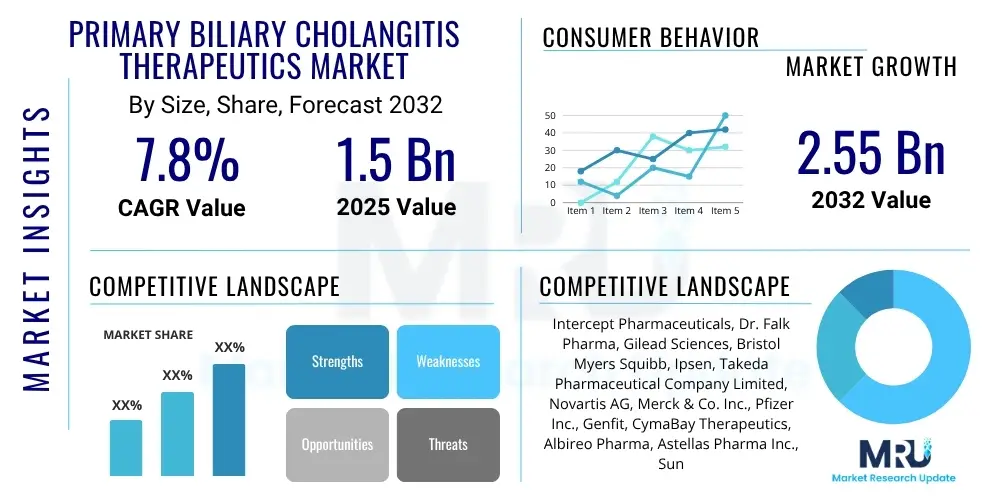
The Primary Biliary Cholangitis Therapeutics Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2025 and 2032. The market is estimated at $1.5 Billion in 2025 and is projected to reach $2.55 Billion by the end of the forecast period in 2032.
Primary Biliary Cholangitis Therapeutics Market introduction
The Primary Biliary Cholangitis Therapeutics Market encompasses a range of pharmaceutical interventions aimed at managing and treating Primary Biliary Cholangitis (PBC), a chronic, progressive autoimmune liver disease characterized by the destruction of small bile ducts within the liver. This condition, if left untreated, can lead to cirrhosis, liver failure, and necessitate liver transplantation. Current therapeutic approaches focus on slowing disease progression, alleviating symptoms such as debilitating fatigue and pruritus, and improving patient quality of life. The market includes established first-line therapies like ursodeoxycholic acid (UDCA), as well as more recent second-line agents such as obeticholic acid (OCA) for patients who do not respond adequately to UDCA or are intolerant to it, alongside a robust pipeline of emerging drug candidates.
The primary products in this market are drugs designed to modulate the immune response, enhance bile flow, or reduce liver inflammation and fibrosis. Major applications are found in the long-term management of adult patients diagnosed with PBC, particularly those experiencing active disease progression or severe symptoms. These therapeutics are administered in various clinical settings, including hospitals, specialty clinics, and increasingly, through home care with oral medications. The benefits derived from these treatments are significant, ranging from improved biochemical markers of liver function, such as alkaline phosphatase and bilirubin levels, to the reduction in the incidence of liver-related complications and mortality, ultimately delaying the need for liver transplantation. Furthermore, effective symptom management profoundly enhances patients' daily lives and functional capabilities.
Key driving factors for the expansion of the Primary Biliary Cholangitis Therapeutics Market include the increasing global prevalence of autoimmune diseases, leading to a larger patient pool requiring treatment. Advancements in diagnostic techniques, including improved serological testing and non-invasive imaging, have led to earlier and more accurate diagnosis, thereby expanding the treated population. A growing understanding of PBC pathophysiology has fueled robust research and development activities, resulting in the introduction of novel therapeutic agents with improved efficacy and safety profiles. Moreover, an aging global population, coupled with rising healthcare expenditure and greater awareness among both patients and healthcare providers about PBC, continues to stimulate market growth, emphasizing the need for effective and accessible treatment options.
Primary Biliary Cholangitis Therapeutics Market Executive Summary
The Primary Biliary Cholangitis Therapeutics Market is experiencing dynamic growth driven by an increasing understanding of disease mechanisms and a growing demand for effective treatment options, particularly for patients unresponsive to conventional therapies. Business trends indicate a strong focus on strategic collaborations between pharmaceutical companies and academic institutions to accelerate drug discovery and development, alongside increased investment in orphan drug designations to capitalize on regulatory incentives for rare diseases. There is a palpable shift towards personalized medicine approaches, leveraging biomarkers to identify patient subgroups most likely to benefit from specific treatments, thereby optimizing therapeutic outcomes and market penetration. Furthermore, companies are investing in patient support programs to enhance adherence and improve overall disease management, which strengthens brand loyalty and market position in a competitive landscape.
Regional trends highlight North America and Europe as dominant markets due to high disease prevalence, advanced healthcare infrastructure, high diagnosis rates, and significant healthcare expenditure. These regions also benefit from favorable reimbursement policies and robust research and development ecosystems that foster innovation. The Asia Pacific region is emerging as a rapidly growing market, propelled by increasing awareness, improving diagnostic capabilities, rising disposable incomes, and the expansion of healthcare access. Latin America and the Middle East and Africa regions, while smaller, are expected to exhibit steady growth as healthcare systems develop and access to specialized treatments improves, presenting untapped opportunities for market players to expand their footprint and address unmet medical needs within these developing economies.
Segmentation trends reveal that the Ursodeoxycholic Acid (UDCA) segment continues to be a cornerstone of the market as the established first-line therapy, benefiting from its long-standing efficacy and cost-effectiveness. However, the Obeticholic Acid (OCA) segment is gaining substantial traction, especially for patients with an inadequate response or intolerance to UDCA, demonstrating the growing demand for second-line options. The pipeline for novel therapeutic agents, including fibrates, immunomodulators, and targeted biologics, is expanding, signaling a future market shift towards more specialized and potent treatments for complex patient profiles. Moreover, there is an increasing emphasis on symptom management therapies, particularly for chronic pruritus and fatigue, which significantly impact patient quality of life, indicating a holistic approach to PBC patient care that extends beyond liver function preservation to comprehensive symptomatic relief.
AI Impact Analysis on Primary Biliary Cholangitis Therapeutics Market
User inquiries about AI's influence on the Primary Biliary Cholangitis Therapeutics Market frequently revolve around its potential to revolutionize drug discovery, accelerate clinical trials, and enable highly personalized treatment regimens. There is considerable interest in how AI can identify novel therapeutic targets, predict drug efficacy, and stratify patients for better clinical outcomes. Users also express expectations regarding AI's ability to improve diagnostic accuracy and early detection, thereby allowing for timely intervention. Concerns often touch upon data privacy, the validation of AI models in a clinical setting, and the ethical implications of AI-driven healthcare decisions. Overall, users anticipate that AI will significantly enhance efficiency, reduce costs, and lead to more effective and individualized therapies for PBC, addressing current unmet needs and transforming patient care pathways.
- AI can significantly accelerate the identification of novel therapeutic targets by analyzing vast datasets of genomic, proteomic, and clinical information, uncovering complex disease pathways specific to PBC and fostering the development of precision medicines.
- Artificial intelligence algorithms are being utilized to optimize clinical trial design, including patient recruitment and stratification, thereby reducing trial duration and costs, while also improving the chances of success for new PBC therapies.
- AI-driven predictive analytics tools can forecast disease progression in individual patients, enabling earlier intervention and the customization of treatment plans based on a patient's unique biological and clinical profile, enhancing the efficacy of PBC management.
- The integration of machine learning into diagnostic tools can improve the accuracy and speed of PBC diagnosis by analyzing imaging data and biochemical markers, leading to earlier therapeutic initiation and potentially preventing advanced liver damage.
- AI supports the development of personalized medicine by analyzing individual patient data to predict responses to specific drugs, thereby tailoring treatment strategies to minimize side effects and maximize therapeutic benefits for PBC patients.
DRO & Impact Forces Of Primary Biliary Cholangitis Therapeutics Market
The Primary Biliary Cholangitis Therapeutics Market is shaped by a complex interplay of driving forces, restraining factors, and emerging opportunities. Key drivers include the escalating global prevalence of Primary Biliary Cholangitis, which continues to expand the patient pool requiring long-term treatment. Substantial investments in pharmaceutical research and development, particularly for orphan drugs, are yielding novel compounds with improved efficacy and reduced side effect profiles. Additionally, favorable regulatory environments in major markets, offering incentives such as accelerated approval pathways and extended market exclusivity for rare disease treatments, encourage innovation and commercialization of new PBC therapies. The persistent unmet medical needs in patients who are non-responsive to or intolerant of existing first-line treatments further fuel the demand for advanced therapeutic solutions, propelling market expansion.
Despite these growth drivers, several restraints challenge market expansion. The high cost associated with novel and advanced PBC therapies often poses a significant barrier to patient access and reimbursement, particularly in healthcare systems with budget constraints or in developing regions. Furthermore, the availability and off-label use of generic drugs, while offering cost-effective alternatives, can sometimes limit the market share and revenue potential of branded medications. The presence of side effects associated with current treatments, such as pruritus or gastrointestinal issues, can lead to patient non-adherence or discontinuation, necessitating continuous efforts to improve drug tolerability. Moreover, the lack of a definitive cure for PBC means that all existing treatments aim to manage the disease and its progression, rather than eradicate it, which inherently limits the therapeutic endpoints and patient expectations.
Opportunities for growth are abundant within the Primary Biliary Cholangitis Therapeutics Market. The development of highly targeted therapies, including novel small molecules and biologics that address specific pathophysiological pathways, represents a significant avenue for future expansion. Exploring combination therapies, leveraging synergistic effects of different drug classes, could offer enhanced efficacy for complex patient cases. The advent of gene therapies and other advanced biotechnological interventions holds promise for transformative treatments in the long term. Moreover, expansion into emerging markets, characterized by improving healthcare infrastructure and increasing patient awareness, offers substantial untapped potential. Continued advancements in diagnostic tools that enable earlier and more precise diagnosis will also broaden the eligible patient population, further stimulating demand for therapeutic interventions and driving market growth.
Segmentation Analysis
The Primary Biliary Cholangitis Therapeutics Market is meticulously segmented to provide a granular understanding of its diverse components, offering insights into treatment preferences, distribution dynamics, and end-user adoption. This segmentation helps stakeholders identify key growth areas, understand competitive landscapes, and formulate targeted market strategies. The market is primarily analyzed across key dimensions including drug type, which categorizes treatments based on their active pharmaceutical ingredients and mechanisms of action; distribution channel, detailing the various routes through which these therapeutics reach patients; and end-user, differentiating the primary consumers of these specialized treatments within the healthcare ecosystem. Each segment reflects unique characteristics and contributes distinctly to the overall market valuation and trajectory.
- By Drug Type
- Ursodeoxycholic Acid (UDCA): The first-line therapeutic standard, widely used for its bile acid modification properties.
- Obeticholic Acid (OCA): A second-line treatment for UDCA non-responders, known for its farnesoid X receptor (FXR) agonism.
- Fibrates: Often used off-label, particularly bezafibrate, to improve liver biochemistry in some PBC patients, either alone or in combination.
- Corticosteroids: Employed in specific circumstances, such as autoimmune hepatitis overlap syndromes, to reduce inflammation.
- Immunosuppressants: Less common but used in specific cases, primarily for managing concomitant autoimmune conditions.
- Emerging Biologics and Novel Therapies: A pipeline of new drugs targeting specific inflammatory pathways, bile acid metabolism, or fibrosis.
- By Distribution Channel
- Hospital Pharmacies: Primary channel for initial prescriptions and for patients requiring inpatient care or close monitoring.
- Retail Pharmacies: Key channel for outpatient prescriptions, offering convenience and broad accessibility for chronic medication management.
- Online Pharmacies: Growing channel, providing increasing accessibility, often with competitive pricing and home delivery options, enhancing patient convenience.
- By End-User
- Hospitals: Major end-users, particularly for patients with advanced PBC, complications, or those initiating complex therapies under specialist supervision.
- Specialty Clinics: Liver centers and gastroenterology clinics, where most PBC patients receive ongoing diagnosis, treatment, and management by specialists.
- Ambulatory Surgical Centers: Less direct for PBC therapeutics, but related to procedures for complications of liver disease.
Value Chain Analysis For Primary Biliary Cholangitis Therapeutics Market
The value chain for the Primary Biliary Cholangitis Therapeutics Market is intricate, beginning with extensive upstream activities focused on scientific discovery and development. This initial phase involves pharmaceutical companies and academic research institutions investing heavily in fundamental research to understand the pathophysiology of PBC, identify potential drug targets, and synthesize novel compounds. This stage encompasses preclinical research, including in vitro and in vivo studies, to assess drug efficacy and safety. Furthermore, the manufacturing of active pharmaceutical ingredients (APIs) is a critical upstream component, requiring specialized facilities and stringent quality control to ensure purity and potency. These activities are highly capital-intensive and subject to rigorous regulatory oversight, forming the foundational bedrock upon which therapeutic advancements are built.
Moving downstream, the value chain encompasses drug formulation, where APIs are converted into finished dosage forms suitable for patient administration, such as tablets or capsules. This is followed by the extensive and often protracted process of clinical trials, which involves multiple phases to evaluate safety, dosage, and efficacy in human subjects, culminating in regulatory approval from health authorities like the FDA or EMA. Post-approval, significant investment is directed towards marketing and sales strategies, including medical education for healthcare professionals, direct-to-consumer advertising where permitted, and engagement with patient advocacy groups. This downstream segment also involves pharmacovigilance, ensuring ongoing monitoring of drug safety and efficacy in the real-world setting, and continuously refining treatment guidelines based on accumulated clinical experience and data.
The distribution channel plays a pivotal role in connecting pharmaceutical manufacturers with end-users, ensuring that PBC therapeutics are accessible to patients. This involves a network of wholesalers, distributors, and logistics providers responsible for the storage, transportation, and inventory management of these specialized medications. Distribution can be direct, where manufacturers establish their own sales forces to engage directly with large hospital systems or specialty clinics, often for high-value or complex therapies. Alternatively, indirect distribution leverages established third-party distributors and wholesalers who then supply to retail pharmacies, hospital pharmacies, and specialty clinics. Online pharmacies are increasingly forming a critical part of the indirect channel, offering convenience and potentially broader reach, especially for patients in remote areas, thereby diversifying access points and impacting market dynamics significantly.
Primary Biliary Cholangitis Therapeutics Market Potential Customers
The primary potential customers and end-users of Primary Biliary Cholangitis (PBC) therapeutics are multifaceted, encompassing the entire spectrum of the healthcare ecosystem involved in chronic liver disease management. At the forefront are hepatologists and gastroenterologists, who specialize in liver and digestive disorders and are responsible for diagnosing, treating, and managing PBC patients. These medical professionals drive prescription patterns based on clinical guidelines, patient response, and the latest research findings. Beyond individual practitioners, institutional buyers such as hospitals, particularly those with specialized liver units or transplant centers, represent a significant customer segment. These institutions procure PBC drugs for their inpatient populations and outpatient clinics, playing a crucial role in the initial diagnosis and management of severe or complicated cases.
Specialty clinics dedicated to liver diseases or autoimmune disorders are another vital customer group. These clinics often provide comprehensive, long-term care for PBC patients, from diagnosis to ongoing therapeutic management and symptom control. They require a consistent supply of a broad range of PBC medications to cater to diverse patient needs, including those who are non-responsive to first-line treatments or require combination therapies. Moreover, national healthcare systems, insurance providers, and government procurement agencies act as large-scale buyers, particularly in countries with universal healthcare coverage. These entities negotiate bulk purchasing agreements and establish formularies that dictate which PBC therapeutics are reimbursed and accessible to their covered populations, profoundly influencing market demand and product adoption rates across entire patient demographics.
Ultimately, individual patients, acting under the guidance of their physicians, are the final beneficiaries and, in a sense, the ultimate customers of PBC therapeutics. Their choices, often influenced by treatment efficacy, tolerability, cost, and insurance coverage, dictate the success and uptake of different medications. Patient advocacy groups also play an increasingly important role, educating patients and advocating for access to novel and effective treatments, thereby indirectly influencing market demand. As a chronic condition, PBC requires lifelong management, making patient adherence and long-term satisfaction critical factors. Therefore, pharmaceutical companies focus on developing therapies that not only improve clinical outcomes but also offer a favorable safety profile and ease of administration, directly impacting patient preference and loyalty, and consequently, the sustained demand within the market.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $1.5 Billion |
| Market Forecast in 2032 | $2.55 Billion |
| Growth Rate | CAGR 7.8% |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Intercept Pharmaceuticals, Dr. Falk Pharma, Gilead Sciences, Bristol Myers Squibb, Ipsen, Takeda Pharmaceutical Company Limited, Novartis AG, Merck & Co. Inc., Pfizer Inc., Genfit, CymaBay Therapeutics, Albireo Pharma, Astellas Pharma Inc., Sun Pharmaceutical Industries Ltd., Zydus Cadila, Roche Holding AG, GlaxoSmithKline plc, Sanofi S.A., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Primary Biliary Cholangitis Therapeutics Market Key Technology Landscape
The technological landscape of the Primary Biliary Cholangitis Therapeutics Market is rapidly evolving, driven by innovations across various scientific disciplines aimed at improving diagnostic accuracy, therapeutic efficacy, and patient outcomes. One crucial area is the advancement in molecular diagnostics and biomarker identification. Technologies such as high-throughput sequencing, proteomics, and metabolomics are being utilized to discover novel biomarkers that can predict disease progression, identify patients most likely to respond to specific treatments, and monitor therapeutic responses more effectively. This allows for a more personalized approach to PBC management, moving away from a one-size-fits-all model towards precision medicine where treatments are tailored to individual patient profiles, significantly enhancing the potential for successful intervention and minimizing adverse effects.
Furthermore, significant technological progress is observed in drug discovery and development platforms. High-throughput screening (HTS) and advanced computational modeling, including in silico drug design, are accelerating the identification and optimization of novel chemical entities that can selectively target specific pathways implicated in PBC pathogenesis, such as FXR agonists, PPAR agonists, and immunomodulators. This reduces the time and cost associated with early-stage drug development. Concurrently, innovations in drug delivery systems, such as sustained-release formulations or targeted delivery mechanisms, are enhancing drug bioavailability, reducing dosing frequency, and improving patient adherence, which are critical for the long-term management of a chronic condition like PBC. These technological strides are fundamentally reshaping the pipeline of future PBC therapeutics.
The integration of artificial intelligence (AI) and machine learning (ML) is another transformative technological trend within the PBC therapeutics market. AI and ML algorithms are being applied to analyze complex clinical, genomic, and real-world data to identify new drug targets, optimize clinical trial design, and predict patient responses to different treatments with greater accuracy. This not only streamlines the drug development process but also facilitates the repurposing of existing drugs for PBC. Additionally, the development of advanced imaging techniques, such as elastography and specific MRI protocols, combined with AI-powered analysis, offers non-invasive methods for assessing liver fibrosis and disease severity, reducing the need for invasive biopsies. These technological advancements collectively contribute to a more efficient, precise, and patient-centric approach to PBC therapeutics, driving innovation across the entire value chain.
Regional Highlights
- North America, particularly the United States and Canada, represents a dominant force in the Primary Biliary Cholangitis Therapeutics Market, driven by a high prevalence of autoimmune diseases, sophisticated healthcare infrastructure, and significant healthcare expenditure. The region benefits from early and accurate diagnosis rates, robust research and development activities, and the presence of major pharmaceutical companies with strong product pipelines. Favorable reimbursement policies and strong patient advocacy groups also contribute to high adoption rates of advanced PBC therapies. The substantial investment in healthcare innovation and specialized liver centers further solidifies North America's leading position, making it a pivotal market for new drug launches and clinical advancements.
- Europe stands as another prominent market for PBC therapeutics, with countries such as Germany, France, the UK, Italy, and Spain contributing significantly. The region possesses well-established healthcare systems, a high awareness of chronic liver diseases, and a strong regulatory framework that supports pharmaceutical innovation and market access. Growing investment in research and development, particularly in personalized medicine approaches, coupled with an aging population susceptible to chronic conditions like PBC, fuels market growth. European nations often have comprehensive national health services that ensure broad patient access to approved treatments, albeit with varying reimbursement criteria and pricing pressures across different countries.
- The Asia Pacific (APAC) region is projected to experience substantial growth in the Primary Biliary Cholangitis Therapeutics Market, attributed to improving healthcare infrastructure, rising disposable incomes, and increasing awareness of liver diseases. Countries like Japan, China, and India are emerging as key markets, driven by a large patient pool, expanding access to diagnostic facilities, and a growing focus on specialty medicine. Government initiatives to enhance healthcare access and affordability, coupled with the entry of global pharmaceutical players into these markets, are accelerating the adoption of advanced PBC therapies. The region also presents significant opportunities for clinical trials due to its large and diverse patient populations.
- Latin America, encompassing countries such as Brazil, Mexico, and Argentina, is an emerging market for PBC therapeutics. Market growth in this region is propelled by increasing investment in healthcare infrastructure, improving diagnostic capabilities, and a rising prevalence of chronic diseases. While facing challenges related to healthcare access and affordability, the region is gradually adopting more advanced treatment protocols as awareness among healthcare professionals and patients grows. International pharmaceutical companies are increasingly looking to penetrate these markets, often through partnerships with local distributors, to address the unmet medical needs of the expanding patient population.
- The Middle East and Africa (MEA) region is also witnessing gradual growth in the PBC therapeutics market. This growth is primarily driven by improvements in healthcare expenditure, increasing health awareness, and the development of specialized medical facilities, particularly in Gulf Cooperation Council (GCC) countries and South Africa. While market penetration for advanced therapies may be slower due to economic disparities and varied healthcare systems, increasing prevalence data and focused efforts by governments to enhance chronic disease management are creating new opportunities. Regional governments and healthcare providers are focusing on international collaborations to bring advanced medical treatments and technologies to their populations, supporting market expansion over the forecast period.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Primary Biliary Cholangitis Therapeutics Market.- Intercept Pharmaceuticals
- Dr. Falk Pharma
- Gilead Sciences
- Bristol Myers Squibb
- Ipsen
- Takeda Pharmaceutical Company Limited
- Novartis AG
- Merck & Co. Inc.
- Pfizer Inc.
- Genfit
- CymaBay Therapeutics
- Albireo Pharma
- Astellas Pharma Inc.
- Sun Pharmaceutical Industries Ltd.
- Zydus Cadila
- Roche Holding AG
- GlaxoSmithKline plc
- Sanofi S.A.
- Eisai Co. Ltd.
- Mitsubishi Tanabe Pharma Corporation
Frequently Asked Questions
What is Primary Biliary Cholangitis (PBC)?
Primary Biliary Cholangitis (PBC) is a chronic autoimmune liver disease characterized by the progressive destruction of small bile ducts within the liver, leading to the accumulation of bile and subsequent inflammation and scarring. If left untreated, PBC can advance to cirrhosis, liver failure, and necessitate a liver transplant. It predominantly affects women and is often diagnosed in middle age, with symptoms including fatigue, pruritus, and jaundice as the disease progresses.
What are the main therapeutic approaches for Primary Biliary Cholangitis?
The main therapeutic approaches for Primary Biliary Cholangitis (PBC) aim to slow disease progression and manage symptoms. Ursodeoxycholic acid (UDCA) is the established first-line therapy, which helps to improve bile flow and reduce liver inflammation. For patients who do not respond adequately to UDCA or are intolerant to it, obeticholic acid (OCA) is a second-line treatment option, acting as a farnesoid X receptor (FXR) agonist to regulate bile acid synthesis. Emerging therapies and combination approaches are continually being investigated to provide more effective solutions, particularly for patients with unmet medical needs.
Are there novel therapies currently in development for PBC?
Yes, there is a robust pipeline of novel therapies in development for Primary Biliary Cholangitis (PBC), reflecting ongoing research into the disease's complex pathophysiology. These emerging treatments often target specific mechanisms involved in bile acid regulation, inflammation, or fibrosis. Examples include new FXR agonists, PPAR agonists, apical sodium-dependent bile acid transporter (ASBT) inhibitors, and various immunomodulatory agents. The goal of these novel therapies is to offer improved efficacy, better tolerability, and additional options for patients who do not respond well to existing treatments, ultimately aiming to halt or reverse liver damage.
How is Primary Biliary Cholangitis typically diagnosed?
Primary Biliary Cholangitis (PBC) is typically diagnosed through a combination of clinical evaluation, blood tests, and sometimes liver imaging or biopsy. Key diagnostic indicators include elevated levels of alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) in blood tests, along with the presence of anti-mitochondrial antibodies (AMA), which are a hallmark of the disease. In some cases, a liver biopsy may be performed to assess the extent of liver damage and confirm the diagnosis, especially when AMA results are ambiguous or to rule out other liver conditions. Non-invasive imaging techniques like elastography can also help assess liver fibrosis.
What are the most common symptoms experienced by individuals with PBC?
The most common symptoms experienced by individuals with Primary Biliary Cholangitis (PBC) include debilitating fatigue and persistent pruritus (itching). Fatigue, often severe, can significantly impact quality of life and daily activities, while pruritus can range from mild to intense and can be generalized or localized. Other potential symptoms, particularly as the disease progresses, may include dry eyes and mouth, joint pain, abdominal discomfort, and jaundice (yellowing of the skin and eyes) due to impaired bile flow. Early diagnosis and treatment are crucial to manage these symptoms and slow the disease's progression.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
