Prosthetic Heart Valves Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429751 | Date : Nov, 2025 | Pages : 243 | Region : Global | Publisher : MRU
Prosthetic Heart Valves Market Size
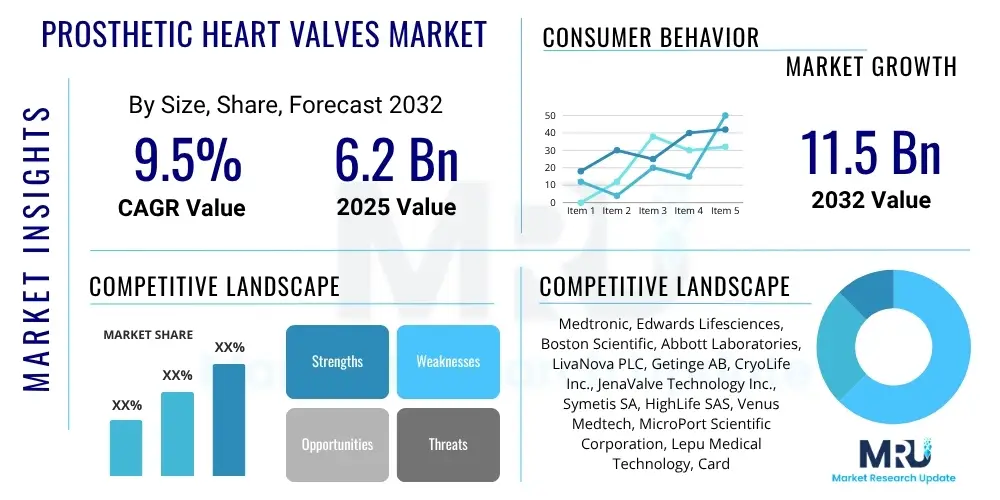
The Prosthetic Heart Valves Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2025 and 2032. The market is estimated at USD 6.2 Billion in 2025 and is projected to reach USD 11.5 Billion by the end of the forecast period in 2032.
Prosthetic Heart Valves Market introduction
The prosthetic heart valves market encompasses devices used to replace or repair diseased heart valves, addressing conditions like stenosis (narrowing) and regurgitation (leaking). These valves restore normal blood flow, alleviating symptoms and improving patient prognosis. Products range from mechanical valves, known for their durability but requiring lifelong anticoagulation, to bioprosthetic valves derived from animal tissue, which offer a more natural hemodynamic profile but have a limited lifespan. Innovations such as transcatheter aortic valve replacement (TAVR) have revolutionized treatment by offering minimally invasive options for patients previously deemed high-risk for open-heart surgery, significantly expanding the addressable market.
Major applications of prosthetic heart valves primarily involve the treatment of valvular heart diseases affecting the aortic, mitral, pulmonary, and tricuspid valves. The benefits to patients are profound, including improved cardiac function, reduced symptoms such as shortness of breath and fatigue, increased life expectancy, and enhanced quality of life. The market is propelled by several key driving factors, notably the global aging population, which is more susceptible to degenerative valvular conditions, and the rising prevalence of cardiovascular diseases. Continuous advancements in material science, surgical techniques, and transcatheter technologies further stimulate market expansion by offering safer, more effective, and less invasive treatment options, alongside increasing healthcare expenditure and awareness regarding early diagnosis and intervention.
Prosthetic Heart Valves Market Executive Summary
The prosthetic heart valves market is experiencing robust growth, driven by a confluence of demographic shifts and technological advancements. Business trends indicate a strong focus on research and development for less invasive procedures, particularly within the transcatheter valve segment, and strategic mergers and acquisitions to consolidate market share and expand product portfolios. Leading companies are investing heavily in improving valve durability, enhancing biocompatibility, and developing patient-specific solutions, reflecting a move towards personalized medicine. The competitive landscape is characterized by a mix of established global players and innovative startups, all vying for differentiation through clinical outcomes and technological superiority.
Regional trends highlight North America and Europe as mature markets with high adoption rates due to advanced healthcare infrastructure and favorable reimbursement policies, while the Asia Pacific region is emerging as a significant growth engine. This growth in APAC is fueled by increasing healthcare expenditure, a large and aging patient pool, improving diagnostic capabilities, and a rising awareness of valvular heart diseases. Segment trends confirm the dominance of bioprosthetic valves, especially the transcatheter segment, over mechanical valves, owing to the increasing preference for minimally invasive procedures and the expanding indications for TAVR in lower-risk patients. The market is also seeing a shift towards technologically advanced materials that reduce the risk of calcification and thrombosis, improving long-term patient outcomes across all valve types and contributing to overall market dynamism.
AI Impact Analysis on Prosthetic Heart Valves Market
The integration of Artificial Intelligence (AI) in the prosthetic heart valves market is a topic of significant interest, with users frequently querying its role in enhancing diagnosis, optimizing treatment strategies, and personalizing device design. Key themes revolve around AI's potential to improve the accuracy of pre-operative imaging analysis, predict patient outcomes, and guide surgical planning, particularly for complex TAVR procedures. Concerns often center on data privacy, regulatory hurdles for AI-powered devices, the cost implications of implementing AI solutions, and ensuring the reliability and interpretability of AI algorithms in critical medical applications. Users expect AI to reduce human error, streamline workflows, and ultimately lead to more effective and safer patient care, while also seeking assurances regarding ethical considerations and data security.
- AI enhances diagnostic accuracy through advanced image analysis (echocardiography, CT scans), identifying subtle valvular abnormalities.
- Predictive analytics powered by AI aids in patient selection for specific valve types and procedures, optimizing treatment pathways.
- AI assists in personalized valve design and sizing, improving fit and reducing complications.
- Machine learning algorithms can forecast the durability of prosthetic valves and predict potential complications like thrombosis or calcification.
- AI supports robotic-assisted surgical systems for increased precision and reduced invasiveness during valve implantation.
- Optimizes post-operative care by monitoring patient data, flagging adverse events, and guiding rehabilitation protocols.
- Facilitates drug discovery for anti-calcification treatments by analyzing large datasets.
- Streamlines clinical trials by identifying suitable patient cohorts and analyzing trial data more efficiently.
DRO & Impact Forces Of Prosthetic Heart Valves Market
The Prosthetic Heart Valves Market is profoundly influenced by a complex interplay of drivers, restraints, opportunities, and broader impact forces. Key drivers include the escalating global prevalence of valvular heart diseases, largely attributed to an aging population and lifestyle-related factors. Technological advancements, particularly in minimally invasive transcatheter procedures like TAVR, and the development of more durable and biocompatible materials, significantly propel market growth. Increasing healthcare expenditure worldwide, coupled with greater awareness about cardiovascular health and early diagnosis, further stimulates demand. These factors collectively create a robust environment for market expansion, pushing for continuous innovation in valve design and surgical approaches to meet growing patient needs.
However, the market also faces considerable restraints, such as the high cost associated with both the prosthetic valves themselves and the complex surgical or interventional procedures required for implantation. Stringent regulatory approval processes, which are necessary to ensure device safety and efficacy, can delay market entry for new innovations. Furthermore, the risk of post-operative complications, including thrombosis, infection, and paravalvular leakage, remains a concern for both patients and clinicians. Limited access to advanced cardiac care in developing regions and a shortage of skilled interventional cardiologists and cardiac surgeons also pose significant barriers to market penetration and growth. Balancing these restraints with the market's inherent growth drivers is crucial for sustainable development.
Opportunities within the prosthetic heart valves market are abundant, especially in emerging economies where healthcare infrastructure is improving and there is a large underserved patient population. The ongoing trend towards personalized medicine and patient-specific valve solutions presents a substantial avenue for innovation. The integration of artificial intelligence and machine learning in diagnostic imaging, surgical planning, and post-operative monitoring offers avenues for enhanced precision and improved outcomes. Additionally, the development of novel biomaterials with enhanced anti-thrombotic and anti-calcification properties, along with entirely new valve designs, continues to open new frontiers for market expansion and patient benefit. The market is also heavily shaped by various impact forces, including evolving regulatory landscapes, global economic conditions, the demographics of aging populations, and public health policies that prioritize cardiovascular health, all of which dynamically influence market adoption and development.
Segmentation Analysis
The prosthetic heart valves market is segmented based on various critical attributes, providing a granular view of its structure and dynamics. These segments help in understanding market penetration, identifying growth areas, and evaluating competitive landscapes across different product types, materials, and end-user applications. Each segment possesses distinct characteristics influenced by technological innovation, clinical guidelines, patient demographics, and regional healthcare infrastructure. Analyzing these segments is essential for stakeholders to formulate targeted strategies, optimize resource allocation, and address specific market demands effectively.
- By Product Type:
- Mechanical Heart Valves
- Bioprosthetic Heart Valves
- Transcatheter Heart Valves (TAVR/TAVI)
- Surgically Implanted Heart Valves (SAVR)
- By Material:
- Nitinol
- Bovine Pericardial Tissue
- Porcine Tissue
- Carbon
- Other Metals (e.g., Titanium)
- Polymers
- By End User:
- Hospitals
- Ambulatory Surgical Centers
- Cardiac Catheterization Labs
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America
- Middle East & Africa (MEA)
Value Chain Analysis For Prosthetic Heart Valves Market
The value chain for the prosthetic heart valves market begins with upstream activities involving the sourcing and processing of highly specialized raw materials. This includes advanced biomaterials like porcine or bovine pericardial tissue, sophisticated polymers, and specific metal alloys such as nitinol and titanium for frames and structural components. Suppliers in this phase are critical for providing high-quality, biocompatible, and durable materials that meet stringent medical standards. Research and development also represent a significant upstream activity, focusing on novel valve designs, material science advancements, and manufacturing processes to enhance device performance and reduce complications, ensuring that products adhere to rigorous safety and efficacy requirements before moving to the manufacturing stage.
Moving through the value chain, manufacturing involves precision engineering, assembly, and sterilization of the prosthetic valves, often in highly regulated cleanroom environments. This stage requires specialized expertise and significant capital investment due to the complexity and critical nature of the devices. After manufacturing, the products enter the distribution channel, which can be direct or indirect. Direct distribution involves manufacturers selling directly to large hospital networks or specialized cardiac centers, often leveraging their own sales force and clinical support teams to provide product training and technical assistance. Indirect distribution relies on third-party distributors, often specialized in medical devices, who manage logistics, warehousing, and regional sales, particularly valuable in reaching smaller healthcare facilities or geographically dispersed markets.
Downstream analysis focuses on the end-users and post-market activities. The primary end-users are hospitals, specialized cardiac clinics, and ambulatory surgical centers where valve replacement procedures are performed. These institutions, along with interventional cardiologists and cardiac surgeons, are pivotal in the adoption and successful implantation of prosthetic valves. Post-market surveillance, patient follow-up, and continuous feedback collection are crucial for improving product safety, identifying long-term outcomes, and informing future design iterations. This comprehensive value chain, from raw material sourcing to patient care, emphasizes the interconnectedness of various stakeholders and the critical importance of quality control, regulatory compliance, and continuous innovation at every stage to ensure patient safety and market growth.
Prosthetic Heart Valves Market Potential Customers
The primary potential customers and end-users of prosthetic heart valves are patients diagnosed with valvular heart diseases, including aortic stenosis, mitral regurgitation, or other conditions requiring valve repair or replacement. This demographic primarily includes an aging population, as degenerative valvular conditions become more prevalent with age, as well as individuals with congenital heart defects or those who have suffered from rheumatic fever, which can damage heart valves. Beyond the individual patient, the key decision-makers and purchasers of these devices are healthcare providers and institutions within the cardiovascular care ecosystem.
Specifically, cardiologists, cardiac surgeons, and interventional cardiologists are crucial in identifying suitable candidates for prosthetic valve implantation and selecting the appropriate device based on patient-specific factors, clinical guidelines, and technological advancements. Hospitals, specialized cardiac centers, and ambulatory surgical centers represent the institutional buyers, as they stock and utilize these devices for surgical and transcatheter procedures. These facilities often make purchasing decisions based on clinical outcomes, product efficacy, cost-effectiveness, and the availability of advanced technologies, ensuring they can offer the best possible care to their patients. The ecosystem also includes procurement departments and group purchasing organizations that negotiate favorable terms for healthcare systems, making them indirect, yet significant, customers in the market.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 6.2 Billion |
| Market Forecast in 2032 | USD 11.5 Billion |
| Growth Rate | 9.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, Edwards Lifesciences, Boston Scientific, Abbott Laboratories, LivaNova PLC, Getinge AB, CryoLife Inc., JenaValve Technology Inc., Symetis SA, HighLife SAS, Venus Medtech, MicroPort Scientific Corporation, Lepu Medical Technology, Cardiac Dimensions, NuVera Medical |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Prosthetic Heart Valves Market Key Technology Landscape
The prosthetic heart valves market is characterized by a dynamic and continuously evolving technology landscape, primarily driven by the imperative to enhance patient outcomes, minimize invasiveness, and improve valve durability. Transcatheter Aortic Valve Replacement (TAVR) remains a cornerstone technology, offering a minimally invasive alternative for patients at high surgical risk, and its indications are steadily expanding to intermediate and lower-risk populations. This technology involves intricate delivery systems and highly sophisticated valve designs, often incorporating self-expanding or balloon-expandable nitinol frames and advanced tissue leaflets, signifying a major shift from traditional open-heart surgery.
Beyond TAVR, significant advancements are being made in biomaterials science, focusing on developing more durable and biocompatible materials for both mechanical and bioprosthetic valves. Research includes novel anti-calcification treatments for tissue valves to prolong their lifespan, and innovative coatings for mechanical valves to reduce thrombogenicity, thereby mitigating the need for lifelong anticoagulation in some cases. The emergence of 3D printing and advanced imaging techniques is facilitating the creation of patient-specific valve designs and surgical guides, enabling a more precise and personalized approach to valve replacement. Furthermore, the integration of artificial intelligence and machine learning is increasingly employed in pre-operative planning, intra-operative guidance, and post-operative monitoring to optimize procedural success and predict long-term patient results, further revolutionizing the field.
Regional Highlights
- North America: This region holds a significant share of the prosthetic heart valves market, driven by advanced healthcare infrastructure, high prevalence of cardiovascular diseases, robust reimbursement policies, and early adoption of innovative technologies like TAVR. The U.S. remains a dominant country due to substantial R&D investment and a high number of complex cardiac procedures.
- Europe: A mature market characterized by well-established healthcare systems, an aging population, and increasing awareness of valvular heart conditions. Countries like Germany, France, and the UK are key contributors, benefiting from strong clinical research and government initiatives promoting advanced medical treatments.
- Asia Pacific (APAC): Expected to exhibit the highest growth rate during the forecast period. This growth is fueled by a large and expanding geriatric population, improving healthcare infrastructure, rising disposable incomes, and increasing medical tourism. China and India are emerging as critical markets due to their vast patient pools and growing investments in healthcare facilities.
- Latin America: This region demonstrates steady growth, supported by improving economic conditions, expanding healthcare access, and a gradual increase in the adoption of advanced medical technologies. Brazil and Mexico are leading countries, with efforts to enhance cardiac care facilities.
- Middle East & Africa (MEA): This region is an emerging market, with growth driven by increasing healthcare expenditure, a rising prevalence of non-communicable diseases including CVD, and improvements in medical tourism infrastructure, particularly in countries like Saudi Arabia and the UAE.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Prosthetic Heart Valves Market.- Medtronic
- Edwards Lifesciences
- Boston Scientific
- Abbott Laboratories
- LivaNova PLC
- Getinge AB
- CryoLife Inc.
- JenaValve Technology Inc.
- Symetis SA
- HighLife SAS
- Venus Medtech
- MicroPort Scientific Corporation
- Lepu Medical Technology
- Cardiac Dimensions
- NuVera Medical
- Valtec SA
- Sorin Group
- Labcor Laboratorios Ltda
- Foldax Inc.
- CardiAQ Valve Technologies (acquired by Edwards Lifesciences)
Frequently Asked Questions
What are the main types of prosthetic heart valves?
The two main types are mechanical valves, which are highly durable but require lifelong blood thinners, and bioprosthetic (tissue) valves, derived from animal tissue, offering a more natural blood flow but with a limited lifespan. Transcatheter valves (TAVR) are a type of bioprosthetic valve implanted minimally invasively.
How long do prosthetic heart valves last?
Mechanical valves typically last 20-30 years or more. Bioprosthetic valves usually last 10-15 years, though advancements are extending their durability. Lifespan can vary based on patient factors, valve type, and lifestyle.
What are the risks associated with heart valve replacement surgery?
Risks include infection, bleeding, stroke, heart attack, kidney problems, arrhythmia, and valve dysfunction. Specific risks vary between open-heart surgery and minimally invasive procedures like TAVR, and are evaluated based on individual patient health.
What is TAVR and who is it for?
TAVR (Transcatheter Aortic Valve Replacement) is a minimally invasive procedure to replace a diseased aortic valve by delivering a new valve through a catheter, usually via the leg artery. It is primarily for patients with severe aortic stenosis who are deemed high or intermediate risk for traditional open-heart surgery, with indications expanding to lower-risk patients.
How is AI impacting the development of new heart valves?
AI is impacting heart valve development by enhancing diagnostic accuracy, optimizing patient selection, aiding in personalized valve design and sizing, and improving surgical planning. It also assists in predicting valve durability and potential complications, streamlining research and development processes for next-generation devices.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
