PTA Balloon Catheter Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 429993 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
PTA Balloon Catheter Market Size
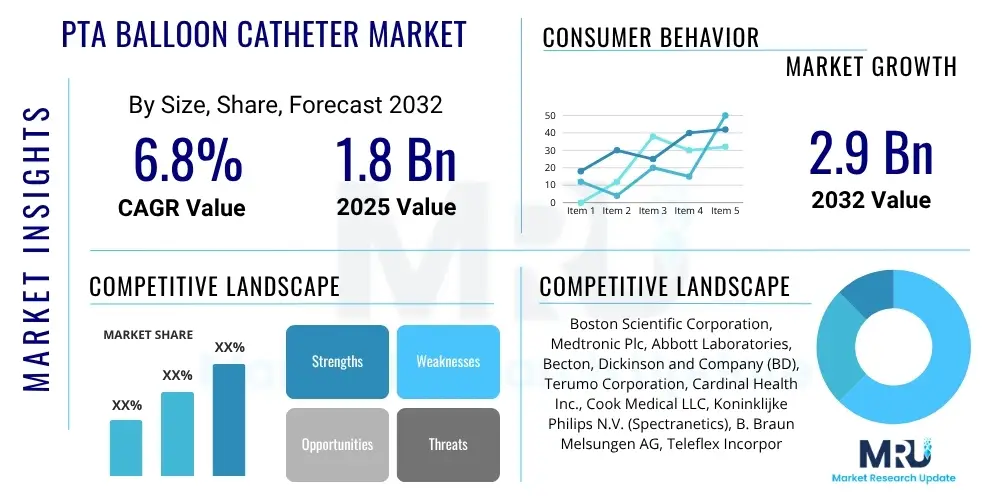
The PTA Balloon Catheter Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 1.8 Billion in 2025 and is projected to reach USD 2.9 Billion by the end of the forecast period in 2032.
PTA Balloon Catheter Market introduction
The Percutaneous Transluminal Angioplasty (PTA) balloon catheter market encompasses medical devices utilized in minimally invasive procedures to widen narrowed or obstructed blood vessels, often due to atherosclerosis or other vascular conditions. These catheters feature an inflatable balloon at their tip, which is guided to the site of the stenosis and inflated to restore proper blood flow. This method is a crucial therapeutic intervention for a range of vascular diseases, offering a less invasive alternative to open surgery, leading to reduced patient recovery times and improved clinical outcomes. The market's evolution is closely tied to advancements in materials science, imaging technologies, and interventional cardiology and radiology techniques.
PTA balloon catheters are integral to treating various vascular occlusions across the body. Their primary application lies in peripheral artery disease (PAD), where blockages affect arteries outside of the heart, particularly in the legs. Beyond PAD, they are also used in coronary artery disease (CAD) for angioplasty, though drug-eluting balloons and stents often accompany these procedures. Furthermore, PTA catheters play a vital role in maintaining the patency of arteriovenous (AV) fistulas and grafts for dialysis access, and in treating venous obstructions. The benefits include a minimally invasive approach, often performed under local anesthesia, with lower risks compared to surgical bypass, and quicker patient discharge, making them a preferred option for many vascular interventions.
The market is primarily driven by the escalating global prevalence of chronic lifestyle diseases such as diabetes, obesity, and hypertension, which are major risk factors for cardiovascular and peripheral vascular conditions. An aging global population, inherently more susceptible to vascular disorders, further fuels demand. Technological advancements, including the development of drug-coated balloons (DCBs), cutting balloons, and scoring balloons, which offer enhanced efficacy in preventing restenosis and treating complex lesions, significantly contribute to market expansion. Favorable reimbursement policies for interventional procedures in developed economies also support the adoption of these advanced catheter technologies.
PTA Balloon Catheter Market Executive Summary
The PTA Balloon Catheter Market is undergoing significant transformation, driven by a confluence of rising chronic disease prevalence, an aging demographic, and continuous technological innovation. Business trends indicate a strong focus on research and development, particularly in drug-coated balloons (DCBs) and specialized catheters designed for specific anatomical challenges and lesion types, such as calcified or highly fibrotic lesions. Strategic mergers, acquisitions, and collaborations are common as companies seek to expand their product portfolios, enhance geographical reach, and integrate advanced technologies. Furthermore, increasing investments in healthcare infrastructure in emerging economies are creating new avenues for market penetration and growth for leading manufacturers.
Regional trends highlight North America and Europe as dominant markets, primarily due to well-established healthcare systems, high awareness of advanced treatment options, and favorable reimbursement scenarios. However, the Asia Pacific region is rapidly emerging as a high-growth market, propelled by its large and aging population, increasing disposable incomes, improving healthcare access, and a growing burden of cardiovascular diseases. Latin America and the Middle East & Africa also present substantial growth opportunities, albeit from a lower base, as healthcare spending rises and medical tourism gains traction. Manufacturers are strategically expanding their distribution networks and local manufacturing capabilities in these regions to capitalize on untapped potential and address unique market demands.
Segmentation trends reveal a notable shift towards advanced product types, with drug-coated balloons experiencing accelerated adoption due to their ability to deliver anti-proliferative drugs directly to the vessel wall, thereby reducing the incidence of restenosis. Standard PTA balloons continue to hold a significant share, but specialized categories like cutting and scoring balloons are gaining traction for treating complex and calcified lesions effectively. In terms of application, peripheral artery disease (PAD) remains the largest segment, but demand for PTA catheters in dialysis access maintenance and venous applications is steadily increasing. Hospitals and specialized cardiac/vascular centers remain the primary end-users, driven by the need for advanced interventional facilities and expert medical personnel.
AI Impact Analysis on PTA Balloon Catheter Market
The integration of Artificial Intelligence (AI) within the PTA Balloon Catheter market elicits diverse user questions, primarily revolving around how AI can enhance diagnostic accuracy, optimize treatment planning, and improve procedural guidance during angioplasty. Users are keenly interested in AI's potential to analyze complex imaging data for precise lesion characterization, predict treatment outcomes, and personalize catheter selection. Concerns often include data privacy, the regulatory pathway for AI-powered medical devices, and the need for robust validation studies to ensure clinical efficacy and safety. Furthermore, there is an expectation for AI to streamline workflows, reduce procedure times, and potentially lower complication rates, transforming the current standard of care.
AI's influence on the PTA Balloon Catheter market is poised to be transformative, moving beyond traditional diagnostic and interventional paradigms. It promises to revolutionize several aspects, from pre-procedural planning to post-procedural assessment, by leveraging advanced algorithms and machine learning. This technological integration is expected to lead to more precise and personalized patient care, offering clinicians enhanced tools for decision-making and operational efficiency. The potential for AI to integrate real-time data during procedures can also significantly improve safety and effectiveness, minimizing human error and optimizing device performance.
The adoption of AI technologies will likely foster a new era of innovation in catheter design and functionality. By analyzing vast datasets of patient anatomies, treatment responses, and device performance, AI can inform the development of next-generation balloons with optimized shapes, sizes, and drug-eluting capabilities. This data-driven approach allows for rapid prototyping and refinement, accelerating the introduction of more effective and safer devices. Consequently, AI is not merely an add-on but a fundamental shift in how PTA balloon catheters are conceived, developed, and utilized in clinical practice.
- AI-enhanced imaging analysis for precise lesion assessment and characterization.
- Predictive analytics to forecast treatment outcomes and identify high-risk patients for restenosis.
- Personalized treatment planning, optimizing catheter size and type based on individual patient anatomy.
- Robotic assistance and navigation systems guided by AI for improved procedural accuracy and reduced radiation exposure.
- Real-time procedural guidance during angioplasty through AI interpretation of live imaging.
- AI-driven post-procedural monitoring for early detection of complications or signs of re-occlusion.
- Accelerated R&D of novel balloon catheter designs through AI-powered simulation and optimization.
DRO & Impact Forces Of PTA Balloon Catheter Market
The PTA Balloon Catheter Market is primarily driven by the increasing global incidence of peripheral artery disease (PAD), coronary artery disease (CAD), and other vascular occlusions, largely attributable to an aging population and the widespread prevalence of risk factors such as diabetes, obesity, and sedentary lifestyles. Continuous technological advancements, particularly in the development of drug-coated balloons (DCBs) that reduce restenosis, and specialized balloons for challenging lesions, significantly expand therapeutic options and adoption. Favorable reimbursement policies in key economies, along with growing awareness among both patients and physicians regarding minimally invasive procedures, further fuel market growth by making these treatments more accessible and appealing. The shift from open surgery to interventional procedures, driven by better patient outcomes, shorter hospital stays, and reduced recovery periods, remains a potent force propelling demand for PTA balloon catheters.
However, the market faces significant restraints, including the high cost associated with advanced interventional procedures and the balloon catheters themselves, which can limit access in price-sensitive markets or for underinsured patient populations. Stringent regulatory approval processes, particularly for novel drug-coated devices, impose considerable R&D costs and delays in market entry, hindering innovation. Furthermore, a scarcity of skilled interventional cardiologists, radiologists, and vascular surgeons, especially in developing regions, poses a challenge to the widespread adoption of these complex procedures. The risk of procedural complications, such as vessel dissection, perforation, or acute vessel closure, although low, also acts as a restraint, prompting continuous efforts in safety enhancement and operator training.
Opportunities for market expansion are abundant, particularly in emerging economies with rapidly improving healthcare infrastructure and growing healthcare expenditure. The development of next-generation drug-eluting technologies and bioresorbable balloons presents significant potential for long-term clinical benefits. Additionally, increasing demand for PTA catheters in new applications, such as neurovascular interventions and specialized pediatric procedures, opens up diversified revenue streams. The growing emphasis on value-based healthcare, which prioritizes effective and cost-efficient treatments, also presents an opportunity for PTA balloon catheters, especially as their long-term efficacy in preventing restenosis continues to improve. These drivers, restraints, and opportunities collectively shape the competitive landscape and strategic direction of the PTA balloon catheter market, exerting significant impact forces on its growth trajectory.
Segmentation Analysis
The PTA Balloon Catheter market is segmented comprehensively across various dimensions, including product type, application, end-user, and material, to provide a granular understanding of market dynamics and identify specific growth areas. This detailed segmentation helps stakeholders analyze market preferences, technological adoption rates, and investment opportunities across different therapeutic areas and clinical settings. Understanding these segments is crucial for strategic planning, product development, and market entry strategies, allowing companies to tailor their offerings to specific needs within the healthcare ecosystem.
- By Product Type
- Standard PTA Balloon Catheters: Basic non-compliant or semi-compliant balloons for general angioplasty.
- Drug-Coated Balloons (DCBs): Balloons coated with anti-proliferative drugs to prevent restenosis.
- Cutting Balloons: Balloons with microtomes designed to score plaques and facilitate vessel dilation.
- Scoring Balloons: Balloons with external wires or ridges to create controlled scoring of plaques.
- High-Pressure Balloons: Designed for resistant or calcified lesions requiring greater inflation force.
- Peripheral Stent Graft Balloons: Used for deploying stent grafts in larger peripheral vessels.
- By Application
- Peripheral Artery Disease (PAD): Treatment of narrowed arteries in the legs, arms, and neck.
- Coronary Artery Disease (CAD): Used in percutaneous coronary interventions (PCI) for heart arteries.
- Dialysis Access Maintenance: Ensuring patency of arteriovenous fistulas and grafts.
- Venous Applications: Treatment of venous obstructions and stenoses.
- Other Applications: Including renal artery stenosis, visceral artery stenosis, and neurovascular applications.
- By End User
- Hospitals: Primary settings for complex and emergency angioplasty procedures.
- Ambulatory Surgical Centers (ASCs): Growing segment due to cost-effectiveness and outpatient convenience.
- Specialty Clinics & Cath Labs: Dedicated facilities for interventional cardiology and radiology.
- By Material
- Nylon: Commonly used for its strength and flexibility.
- Pebax: Known for its excellent trackability and pushability.
- Polyurethane: Offers good elasticity and biocompatibility.
- Other Polymers: Including polyethylene terephthalate (PET) and silicon-based materials.
- By Region
- North America (U.S., Canada, Mexico)
- Europe (Germany, U.K., France, Italy, Spain, Rest of Europe)
- Asia Pacific (China, Japan, India, Australia, South Korea, Rest of APAC)
- Latin America (Brazil, Argentina, Rest of Latin America)
- Middle East & Africa (GCC Countries, South Africa, Rest of MEA)
Value Chain Analysis For PTA Balloon Catheter Market
The value chain for the PTA Balloon Catheter market begins with the upstream segment, which involves the sourcing and production of critical raw materials. This includes specialized polymers such as nylon, Pebax, and polyurethane, which form the balloon and catheter shaft, along with metals like stainless steel or nitinol for guidewires and specific catheter components. Additionally, drug substances for drug-coated balloons, advanced coatings (e.g., hydrophilic, anti-thrombotic), and various lubricants are sourced from specialized chemical and material manufacturers. The quality and availability of these raw materials are paramount, as they directly impact the performance, safety, and regulatory approval of the final product. Suppliers in this segment must adhere to strict quality standards and often work closely with catheter manufacturers to develop bespoke materials that meet the demanding specifications of medical devices.
Following the raw material procurement, the manufacturing process constitutes a crucial part of the value chain. This phase involves complex processes such as extrusion of catheter shafts, balloon forming, precise drug coating, assembly, sterilization, and rigorous quality control testing. Manufacturers invest heavily in R&D to innovate designs, improve balloon compliance and trackability, and enhance drug delivery mechanisms. This segment is characterized by high capital expenditure, advanced technological expertise, and adherence to stringent regulatory frameworks like FDA and CE Mark. Direct manufacturing by large medical device companies is prevalent, alongside partnerships with contract manufacturing organizations (CMOs) specializing in medical device production to optimize costs and leverage specialized capabilities.
The downstream segment of the value chain focuses on the distribution, sales, and end-use of PTA balloon catheters. Products are typically distributed through a combination of direct sales forces, particularly for large key accounts like major hospital networks, and indirect channels involving third-party distributors and wholesalers. These distributors often have established relationships with healthcare providers and possess specialized logistics for handling medical devices. The end-users primarily include hospitals, ambulatory surgical centers, and specialty clinics/cath labs, where interventional cardiologists, radiologists, and vascular surgeons perform the angioplasty procedures. Post-market surveillance, technical support, and ongoing clinical education for healthcare professionals are integral to ensuring proper product usage and patient outcomes, thereby completing the value chain and reinforcing market presence.
PTA Balloon Catheter Market Potential Customers
The primary potential customers for PTA balloon catheters are healthcare facilities and medical professionals specializing in interventional procedures. This largely encompasses hospitals, particularly their cardiology, vascular surgery, and radiology departments, which regularly perform angioplasties to treat conditions such as peripheral artery disease, coronary artery disease, and dialysis access stenosis. These institutions represent the largest segment of end-users due to their infrastructure, patient volume, and capacity for complex procedures, often acting as referral centers for a wide range of vascular interventions. The purchasing decisions within hospitals are influenced by factors like product efficacy, safety profile, cost-effectiveness, and the availability of clinical evidence, alongside the preferences of their leading interventional specialists.
Ambulatory Surgical Centers (ASCs) also represent a rapidly growing segment of potential customers. ASCs offer a cost-effective and convenient alternative to hospital-based procedures for eligible patients, driving an increase in outpatient vascular interventions. As healthcare systems globally shift towards outpatient care models to reduce costs and improve efficiency, the demand for PTA balloon catheters within ASCs is expected to escalate. These centers typically focus on less complex cases but require reliable, high-quality devices that support quick turnaround times and efficient patient flow. Their purchasing decisions are often driven by economic factors and ease of use, alongside clinical performance.
Specialty clinics and dedicated cath labs, whether standalone or affiliated with larger healthcare networks, constitute another significant customer base. These facilities are specifically equipped for diagnostic and interventional cardiovascular and peripheral procedures and are staffed by highly specialized medical personnel. They require a broad range of PTA balloon catheters, including advanced drug-coated and specialized balloons, to address diverse patient needs and complex anatomical challenges. Additionally, government procurement agencies and group purchasing organizations (GPOs) serve as indirect customers, influencing purchasing decisions through large-volume contracts and standardized product selections across multiple healthcare providers. Their collective buying power and focus on cost-efficiency play a crucial role in market access and pricing strategies for manufacturers.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 1.8 Billion |
| Market Forecast in 2032 | USD 2.9 Billion |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Boston Scientific Corporation, Medtronic Plc, Abbott Laboratories, Becton, Dickinson and Company (BD), Terumo Corporation, Cardinal Health Inc., Cook Medical LLC, Koninklijke Philips N.V. (Spectranetics), B. Braun Melsungen AG, Teleflex Incorporated, Biotronik SE & Co. KG, MicroPort Scientific Corporation, Getinge AB (Atrium Medical), Meril Life Sciences Pvt. Ltd., Shenzhen Lifetech Scientific Co., Ltd., Concept Medical Inc., Eurocor GmbH, Translumina GmbH, Opto Medical Technologies, Inari Medical, Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
PTA Balloon Catheter Market Key Technology Landscape
The technological landscape of the PTA Balloon Catheter market is characterized by continuous innovation aimed at improving efficacy, safety, and procedural outcomes. One of the most significant advancements is the development of Drug-Coated Balloons (DCBs), which deliver anti-proliferative agents directly to the vessel wall during angioplasty, inhibiting cell growth and reducing the incidence of restenosis. These balloons typically utilize excipients or specialized coatings to ensure efficient drug transfer, allowing the vessel to remain open longer without the need for a permanent implant like a stent. Further research is focused on optimizing drug formulations, coating durability, and release kinetics to enhance long-term patency rates across various lesion types and anatomical locations.
Beyond drug elution, advancements in balloon material science and design are crucial. High-performance polymers such as Pebax, Nylon, and advanced polyurethanes enable the creation of balloons with improved trackability, pushability, and controlled compliance characteristics. This allows for navigation through tortuous anatomies and precise dilation of calcified or highly fibrotic lesions. Specialized balloon designs, including cutting balloons with micro-blades and scoring balloons with external wires, provide controlled plaque modification, reducing vessel recoil and minimizing injury to the arterial wall. These innovations address the challenges of complex lesion morphology, offering clinicians more tailored tools for challenging interventions and improving the success rates of angioplasty.
Furthermore, the integration of imaging technologies and advanced navigation systems is reshaping the procedural landscape. Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT) provide high-resolution imaging of the vessel lumen and wall, aiding in precise lesion assessment and optimal balloon sizing. Catheters are increasingly being designed with enhanced radiopacity to improve visibility under fluoroscopy, facilitating accurate positioning and deployment. The emergence of steerable catheters and guidewire advancements also contributes to better procedural control and reduced procedural times. Looking ahead, the application of artificial intelligence for predictive analytics, personalized treatment planning, and robotic-assisted interventions holds immense potential to further refine PTA balloon catheter procedures, making them safer, more efficient, and more effective.
Regional Highlights
The global PTA Balloon Catheter market exhibits distinct regional dynamics, influenced by varying healthcare infrastructures, disease prevalence, regulatory frameworks, and economic development levels. North America, particularly the United States, represents a dominant market share due to its advanced healthcare system, high prevalence of cardiovascular and peripheral artery diseases, strong reimbursement policies, and significant investments in medical device research and development. The region benefits from a high adoption rate of innovative technologies and a large pool of skilled interventional specialists. Continuous product launches and strategic collaborations among key players further solidify its leading position, with a strong focus on advanced drug-coated and specialized balloon catheters to address complex patient needs.
Europe also holds a substantial share of the PTA Balloon Catheter market, driven by an aging population highly susceptible to vascular conditions and well-established healthcare systems across Western European countries. Countries like Germany, the UK, France, and Italy are key contributors, characterized by high healthcare expenditure and robust clinical research activities. The adoption of advanced balloon technologies is steadily increasing, supported by government initiatives to improve cardiovascular care and an emphasis on minimally invasive procedures. However, regulatory complexities and pricing pressures in some European markets present unique challenges for manufacturers, necessitating tailored market entry and pricing strategies.
The Asia Pacific (APAC) region is projected to be the fastest-growing market for PTA Balloon Catheters, owing to its massive and rapidly aging population, increasing disposable incomes, and significant improvements in healthcare infrastructure and access. Countries such as China, India, and Japan are at the forefront of this growth, experiencing a surging burden of cardiovascular diseases linked to changing lifestyles and urbanization. Increased awareness of advanced treatment options, coupled with government support for domestic medical device manufacturing and healthcare reforms, is accelerating market expansion. Latin America, the Middle East, and Africa (MEA) regions are also showing promising growth, albeit from a smaller base, driven by rising healthcare investments, growing medical tourism, and a greater emphasis on improving access to advanced medical treatments for vascular conditions.
- North America: Dominant market share due to advanced healthcare infrastructure, high disease prevalence, and strong reimbursement.
- Europe: Significant market, driven by an aging population, established healthcare systems, and increasing adoption of advanced technologies.
- Asia Pacific (APAC): Fastest-growing region, fueled by large patient pool, improving healthcare access, and economic development.
- Latin America: Emerging market with growing healthcare investment and increasing awareness of advanced treatments.
- Middle East & Africa (MEA): Developing market with potential for growth driven by improving healthcare facilities and medical tourism.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the PTA Balloon Catheter Market.- Boston Scientific Corporation
- Medtronic Plc
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- Terumo Corporation
- Cardinal Health Inc.
- Cook Medical LLC
- Koninklijke Philips N.V. (Spectranetics)
- B. Braun Melsungen AG
- Teleflex Incorporated
- Biotronik SE & Co. KG
- MicroPort Scientific Corporation
- Getinge AB (Atrium Medical)
- Meril Life Sciences Pvt. Ltd.
- Shenzhen Lifetech Scientific Co., Ltd.
- Concept Medical Inc.
- Eurocor GmbH
- Translumina GmbH
- Opto Medical Technologies
- Inari Medical, Inc.
Frequently Asked Questions
What is a PTA balloon catheter used for?
A PTA balloon catheter is a medical device used in percutaneous transluminal angioplasty to widen narrowed or blocked blood vessels, primarily in conditions like peripheral artery disease, coronary artery disease, and to maintain dialysis access.
How does a drug-coated balloon catheter (DCB) differ from a standard PTA balloon?
A DCB is coated with an anti-proliferative drug that is transferred to the vessel wall during inflation, helping to prevent restenosis (re-narrowing of the vessel), whereas a standard PTA balloon mechanically dilates the vessel without drug delivery.
What are the primary factors driving the growth of the PTA Balloon Catheter market?
Key drivers include the rising global prevalence of cardiovascular and peripheral artery diseases, an aging population, continuous technological advancements in catheter design and drug-eluting capabilities, and the growing preference for minimally invasive surgical procedures.
What are the major challenges faced by the PTA Balloon Catheter market?
Major challenges include the high cost of advanced interventional procedures, stringent regulatory approval processes, and the shortage of skilled interventional specialists, particularly in developing regions.
Which regions are expected to show significant growth in the PTA Balloon Catheter market?
The Asia Pacific region is anticipated to exhibit the fastest growth due to its large patient population, improving healthcare infrastructure, and increasing awareness of advanced treatment options. North America and Europe remain dominant markets.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
