Radiofrequency Ablation Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 431198 | Date : Nov, 2025 | Pages : 249 | Region : Global | Publisher : MRU
Radiofrequency Ablation Devices Market Size
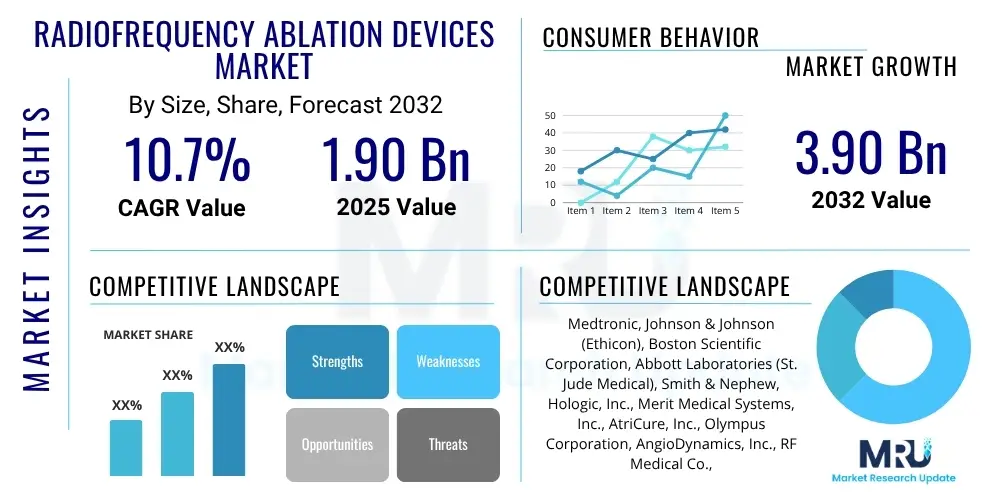
The Radiofrequency Ablation Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 10.7% between 2025 and 2032. The market is estimated at $1.90 Billion in 2025 and is projected to reach $3.90 Billion by the end of the forecast period in 2032.
Radiofrequency Ablation Devices Market introduction
The Radiofrequency Ablation (RFA) Devices Market encompasses a range of medical instruments utilized in minimally invasive procedures to generate heat and destroy targeted tissues. These devices leverage high-frequency alternating currents to produce thermal energy, effectively ablating abnormal cells or nerve tissues. Key product offerings include RF generators, which produce the energy, and various electrodes or catheters designed for precise delivery of this energy to the treatment site. Major applications span across diverse medical fields, including oncology for tumor ablation in organs like the liver, lung, and kidney; pain management for chronic conditions such as back pain and neuropathic pain; cardiology for treating cardiac arrhythmias like atrial fibrillation; and gynecology for conditions like uterine fibroids. The primary benefits of RFA procedures include reduced patient recovery times, minimal scarring, lower risk of complications compared to open surgery, and high efficacy in select conditions.
Driving factors for the robust growth of this market include the increasing global prevalence of chronic diseases, particularly cancer and chronic pain conditions, which often necessitate targeted tissue destruction. The growing demand for minimally invasive surgical interventions due to improved patient outcomes and reduced healthcare costs further propels market expansion. Moreover, significant advancements in RFA technology, such as the development of cooled-tip electrodes, multi-electrode systems, and improved imaging guidance, have enhanced the safety, precision, and applicability of these procedures, making them a preferred treatment option for a broader range of clinical indications. The aging global population, coupled with rising healthcare expenditure and awareness regarding advanced treatment modalities, also contributes significantly to market growth.
Radiofrequency Ablation Devices Market Executive Summary
The Radiofrequency Ablation Devices Market is poised for substantial expansion, driven by a convergence of technological innovation, increasing disease burdens, and a strong preference for less invasive therapeutic options. Business trends indicate a focus on strategic collaborations, mergers, and acquisitions among key players to consolidate market share, diversify product portfolios, and expand geographical reach. Companies are increasingly investing in research and development to introduce advanced devices with enhanced precision, improved safety profiles, and broader application capabilities, aiming to address unmet clinical needs and improve patient outcomes across various therapeutic areas. There is also a growing emphasis on developing user-friendly systems and comprehensive training programs for healthcare professionals to facilitate wider adoption and effective utilization of RFA technologies, particularly in complex procedures.
Regionally, North America and Europe currently dominate the market due to established healthcare infrastructures, high adoption rates of advanced medical technologies, and favorable reimbursement policies for RFA procedures. However, the Asia Pacific region is anticipated to exhibit the fastest growth, propelled by rising healthcare expenditure, increasing medical tourism, a large and aging population, and improving access to advanced medical treatments in emerging economies like China and India. Latin America and the Middle East & Africa also present significant growth opportunities as healthcare infrastructure develops and awareness of minimally invasive procedures increases. Segment-wise, the oncology application segment, particularly for liver and lung tumor ablation, continues to hold the largest market share owing to the high incidence of cancer globally and the proven efficacy of RFA in treating localized tumors. The pain management segment is also witnessing considerable growth, driven by the increasing prevalence of chronic pain conditions and the effectiveness of RFA in providing long-term pain relief. Additionally, the increasing adoption of RFA in cardiology for treating atrial fibrillation is a notable trend, reflecting the expanding versatility and clinical utility of these devices.
AI Impact Analysis on Radiofrequency Ablation Devices Market
User inquiries regarding Artificial Intelligence (AI) in the Radiofrequency Ablation Devices Market frequently revolve around how AI can enhance procedural precision, improve patient selection, optimize treatment planning, and predict outcomes. Users are keen to understand if AI can reduce complications, shorten procedure times, and make RFA therapies more accessible and personalized. Common concerns include the reliability of AI algorithms in critical medical interventions, the integration challenges with existing RFA systems, regulatory hurdles for AI-powered devices, and the ethical implications of autonomous or semi-autonomous AI decision-making in patient care. Expectations are high for AI to revolutionize imaging guidance, assist in real-time monitoring during ablation, and streamline post-procedure analysis, thereby elevating the overall safety and efficacy of RFA treatments.
AI's influence is expected to profoundly transform the Radiofrequency Ablation Devices Market by augmenting precision and personalization in treatment. Through advanced image recognition and machine learning algorithms, AI can analyze complex medical imaging data (CT, MRI, ultrasound) to precisely delineate target lesions and adjacent critical structures, guiding electrode placement with unprecedented accuracy. This capability is particularly crucial in oncology for tumor ablation and in cardiology for mapping intricate cardiac arrhythmias, where precise targeting minimizes damage to healthy tissue and improves therapeutic success rates. Furthermore, AI can process vast amounts of patient data, including medical history, genetics, and physiological responses, to predict individual patient outcomes and identify optimal treatment parameters, leading to highly personalized RFA protocols. This predictive analytics can help clinicians select the most appropriate candidates for RFA, thus maximizing therapeutic benefits and minimizing risks, ultimately enhancing patient safety and clinical efficiency.
The integration of AI also holds immense promise for improving operational efficiency and expanding the accessibility of RFA procedures. AI-powered robotic systems can assist in real-time navigation and manipulation of RFA catheters, potentially reducing procedural variability and operator fatigue. AI algorithms can continuously monitor tissue impedance, temperature, and ablation zone progression during a procedure, providing immediate feedback and enabling dynamic adjustments to energy delivery, thereby preventing complications like incomplete ablation or thermal injury to surrounding tissues. Beyond the operating room, AI can support the development of virtual training platforms for clinicians, accelerating skill acquisition and ensuring consistent procedure quality. The cumulative impact of these advancements is expected to drive greater adoption of RFA therapies, particularly in complex cases and underserved regions, by making them safer, more effective, and more widely available.
- Enhanced Precision and Targeting: AI algorithms analyze imaging for accurate lesion delineation and real-time electrode guidance.
- Personalized Treatment Planning: AI processes patient data to optimize ablation parameters and predict outcomes.
- Robotic-Assisted RFA: AI-powered robotics for automated or semi-automated catheter navigation and energy delivery.
- Real-time Monitoring and Feedback: AI monitors tissue response during ablation to prevent complications.
- Predictive Analytics for Patient Selection: Identifies ideal candidates for RFA based on comprehensive data analysis.
- Streamlined Post-Procedure Analysis: AI assists in assessing ablation completeness and patient recovery.
- Improved Training and Education: Virtual reality and AI simulations for clinician skill development.
DRO & Impact Forces Of Radiofrequency Ablation Devices Market
The Radiofrequency Ablation Devices Market is profoundly influenced by a complex interplay of drivers, restraints, opportunities, and broader impact forces. Key drivers include the escalating global burden of chronic diseases such as cancer, cardiac arrhythmias, and chronic pain, necessitating effective and minimally invasive therapeutic interventions. The increasing preference among both patients and healthcare providers for less invasive procedures, which offer benefits like reduced recovery times, lower complication rates, and diminished healthcare costs, significantly propels market growth. Furthermore, continuous technological advancements, such as the introduction of cooled-tip electrodes, multi-electrode arrays, and advanced imaging integration, are enhancing the safety, efficacy, and precision of RFA procedures, thereby broadening their clinical applicability. Favorable reimbursement policies for RFA treatments in developed economies also play a crucial role in encouraging their adoption. The aging global population, more susceptible to various chronic conditions, further contributes to the expanding patient pool requiring RFA therapies. Moreover, rising healthcare expenditure, particularly in emerging markets, allows for greater investment in advanced medical technologies.
However, several restraints pose challenges to market expansion. The high upfront cost of RFA devices and the associated procedural expenses can limit adoption, especially in resource-constrained settings or regions with less developed healthcare funding mechanisms. Stringent regulatory approval processes for new RFA devices, particularly those incorporating advanced technologies like AI, can delay market entry and increase development costs. A notable challenge is the scarcity of skilled healthcare professionals proficient in performing complex RFA procedures, requiring specialized training and expertise. This limitation can hinder the widespread implementation of RFA, particularly in areas with limited access to advanced medical education. Additionally, the potential for complications, although generally low compared to traditional surgery, such as bleeding, infection, thermal injury to surrounding tissues, or incomplete ablation, can sometimes deter patient and physician adoption. Competitive pressures from alternative treatment modalities, including cryoablation, microwave ablation, and conventional surgery, also necessitate continuous innovation and demonstration of RFA's superior efficacy and safety profiles.
Opportunities for market growth are abundant, particularly in emerging economies where healthcare infrastructure is rapidly improving and access to advanced medical technologies is expanding. These regions represent significant untapped patient populations and growing healthcare expenditure. The ongoing development of advanced technologies, including the integration of AI and robotic assistance into RFA systems, promises to further enhance precision, personalize treatments, and expand the therapeutic scope of RFA. Furthermore, expanding the application of RFA into new therapeutic areas, such as neurological disorders, cosmetic procedures, and benign prostatic hyperplasia, presents avenues for market diversification and growth. Strategic collaborations between device manufacturers, research institutions, and healthcare providers can accelerate product innovation and market penetration. Impact forces, encompassing economic trends, technological shifts, social changes (e.g., increasing health awareness), and regulatory frameworks, collectively shape the market's trajectory, influencing investment decisions, product development, and market access strategies. The shift towards value-based healthcare models also encourages the adoption of cost-effective and outcome-driven treatments like RFA.
Segmentation Analysis
The Radiofrequency Ablation Devices Market is comprehensively segmented across various dimensions to provide a detailed understanding of its structure, dynamics, and growth prospects. These segments categorize the market based on product type, application area, and end-user, reflecting the diverse range of devices available, the breadth of medical conditions treated, and the varied healthcare settings where these procedures are performed. Each segment is critical for analyzing market trends, identifying growth opportunities, and understanding the competitive landscape. The analysis further extends to geographical segmentation, highlighting regional disparities and growth potential based on healthcare infrastructure, disease prevalence, and regulatory environments.
Understanding these segments allows market stakeholders to tailor their strategies, product development, and marketing efforts more effectively. For instance, device manufacturers can focus R&D on specific product types or applications exhibiting high growth, while healthcare providers can optimize their procurement and service offerings based on prevalent end-user demands. The intricate relationships between these segments, such as how technological advancements in generators impact the efficacy of electrodes, or how increased awareness in a specific application drives demand for particular end-users, are crucial for a holistic market perspective. This multi-faceted segmentation ensures that all aspects of the Radiofrequency Ablation Devices Market are thoroughly examined, providing actionable insights for strategic decision-making and fostering sustained market expansion.
- By Product Type:
- Radiofrequency Generators
- Radiofrequency Electrodes
- Reusable Electrodes
- Disposable Electrodes
- Radiofrequency Catheters
- Bipolar Catheters
- Monopolar Catheters
- Radiofrequency Probes
- Accessories
- By Application:
- Oncology
- Liver Cancer
- Lung Cancer
- Kidney Cancer
- Bone Metastasis
- Other Cancers (e.g., Breast Cancer, Pancreatic Cancer)
- Pain Management
- Chronic Back Pain
- Neuropathic Pain
- Osteoarthritis
- Facet Joint Pain
- Trigeminal Neuralgia
- Other Chronic Pain Conditions
- Cardiology
- Atrial Fibrillation (AFib)
- Supraventricular Tachycardia (SVT)
- Ventricular Tachycardia (VT)
- Accessory Pathway Ablation
- Gynecology
- Uterine Fibroids
- Endometriosis
- Adenomyosis
- Urology
- Benign Prostatic Hyperplasia (BPH)
- Kidney Stones
- Renal Cell Carcinoma (early stage)
- Cosmetic
- Wrinkle Reduction
- Skin Tightening
- Cellulite Treatment
- Other Applications (e.g., ENT, Orthopedics, Gastroenterology)
- Oncology
- By End User:
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Specialty Clinics (e.g., Pain Clinics, Oncology Clinics, Cardiology Clinics)
- Academic & Research Institutes
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America
- Middle East and Africa (MEA)
Value Chain Analysis For Radiofrequency Ablation Devices Market
The value chain for the Radiofrequency Ablation Devices Market begins with upstream activities involving the sourcing of highly specialized raw materials and components. This stage includes suppliers of medical-grade metals such as stainless steel and platinum, polymers for catheter construction, sophisticated electronic components for RF generators, and advanced ceramic materials for electrode tips. Research and development activities, often conducted in collaboration with academic institutions and clinical experts, are crucial at this initial phase to innovate and validate new technologies, ensuring device safety and efficacy. Quality control at the component level is paramount to meet stringent medical device standards. The manufacturing process involves the intricate assembly of these components into finished RFA generators, electrodes, catheters, and accessories. This stage requires sterile environments, precision engineering, and rigorous testing protocols to ensure consistent performance, biocompatibility, and compliance with global regulatory requirements.
Further along the value chain, the manufactured devices move into distribution and sales, which represent critical downstream activities. Distribution channels are varied and include direct sales forces, which are often employed by larger manufacturers to target major hospitals and academic medical centers, offering personalized support and training. Indirect channels involve a network of specialized medical device distributors who have established relationships with a broader range of healthcare facilities, including ambulatory surgical centers and specialty clinics, facilitating wider market penetration. E-commerce platforms are also emerging as a supplementary channel, particularly for less complex accessories or for initial product inquiries. Marketing and sales strategies are tailored to educate healthcare professionals about the benefits and technical aspects of RFA devices, often involving clinical demonstrations, conferences, and peer-to-peer training programs. After-sales support, including maintenance, troubleshooting, and continuous education for end-users, plays a vital role in customer satisfaction and repeat business.
The final stage of the value chain involves the end-users, primarily hospitals, ambulatory surgical centers, and specialty clinics, where the RFA devices are utilized for patient treatment. Healthcare providers assess the clinical needs, device capabilities, and cost-effectiveness before procurement. The successful implementation of RFA procedures relies on the skill of the medical practitioners, the integration of RFA systems with imaging modalities, and effective patient management. Feedback from end-users regarding device performance, ease of use, and clinical outcomes is crucial for manufacturers to drive continuous product improvement and innovation, thereby closing the loop in the value chain. Reimbursement policies from government and private payers also significantly influence the adoption and utilization rates of these devices, acting as a critical external factor impacting the economic viability for end-users and manufacturers alike. Overall, the value chain is characterized by high technological intensity, strict regulatory oversight, and a strong emphasis on clinical evidence and post-market surveillance.
Radiofrequency Ablation Devices Market Potential Customers
Potential customers for Radiofrequency Ablation Devices primarily comprise various healthcare institutions and medical professionals who perform minimally invasive procedures for a broad spectrum of conditions. Hospitals, particularly large multi-specialty hospitals, cancer centers, and cardiac care units, represent a significant segment of end-users due to their capacity for high patient volumes, access to advanced imaging technologies, and the presence of diverse medical specialists. These institutions invest in RFA devices for treating complex cases in oncology, cardiology, and pain management, driven by the demand for advanced therapeutic options and the benefits of minimally invasive interventions for patient recovery. The need for robust, reliable, and technologically advanced RFA systems that can be integrated into existing surgical suites and procedural labs is a key purchasing criterion for hospitals.
Ambulatory Surgical Centers (ASCs) constitute another rapidly growing segment of potential customers. ASCs specialize in outpatient procedures, and the minimally invasive nature of RFA makes it an ideal fit for these facilities, offering cost-effective and efficient treatment alternatives to traditional inpatient surgeries. As healthcare shifts towards outpatient settings for appropriate procedures, ASCs are increasingly adopting RFA devices, particularly for pain management, minor oncological ablations, and certain gynecological procedures. Their purchasing decisions are often influenced by device portability, ease of use, and the economic benefits associated with quicker patient turnaround and reduced overhead compared to hospitals. Furthermore, specialty clinics, including dedicated pain management clinics, oncology clinics, and cardiology clinics, represent a focused customer base. These clinics cater to specific patient populations, requiring specialized RFA devices tailored to their niche therapeutic areas, such as specific electrodes for facet joint denervation or specialized catheters for atrial fibrillation ablation. Their buying decisions are driven by the need for highly specialized, effective, and patient-centric solutions. Academic and research institutes also serve as potential customers, utilizing RFA devices for clinical trials, advanced research into new applications, and training the next generation of medical professionals, thereby contributing to the long-term growth and innovation within the market.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | $1.90 Billion |
| Market Forecast in 2032 | $3.90 Billion |
| Growth Rate | 10.7% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic, Johnson & Johnson (Ethicon), Boston Scientific Corporation, Abbott Laboratories (St. Jude Medical), Smith & Nephew, Hologic, Inc., Merit Medical Systems, Inc., AtriCure, Inc., Olympus Corporation, AngioDynamics, Inc., RF Medical Co., Ltd., Baylis Medical Company Inc. (Boston Scientific), STARmed Co., Ltd., Inomed Medizintechnik GmbH, Accuray Incorporated, Brainlab AG, Integra LifeSciences Corporation, ConMed Corporation, Cosman Medical, Inc., Diros Technology Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Radiofrequency Ablation Devices Market Key Technology Landscape
The Radiofrequency Ablation Devices Market is characterized by a dynamic and evolving technology landscape, constantly driven by the quest for enhanced precision, safety, and broader clinical utility. A significant advancement has been the widespread adoption of temperature-controlled RFA systems, which allow for real-time monitoring and adjustment of tissue temperature during ablation, preventing overheating and ensuring the creation of effective and predictable lesion sizes. Cooled-tip electrodes, which circulate saline through the electrode tip, further enhance this control by reducing charring and impedance rise, allowing for larger ablation volumes and deeper penetration while protecting superficial tissues. These innovations significantly improve treatment outcomes, particularly in dense or highly vascularized tissues, and minimize the risk of complications.
Another pivotal technological trend is the development of multi-electrode systems and phased-array RF generators, enabling simultaneous ablation of multiple targets or larger tissue volumes with a single insertion. This reduces procedural time, increases efficiency, and expands the applicability of RFA to more complex anatomical sites. Advanced imaging integration, particularly with ultrasound, CT, and MRI, is crucial for real-time visualization and guidance during RFA procedures. These imaging modalities allow clinicians to precisely position electrodes, monitor the ablation zone, and confirm treatment effectiveness, enhancing both the accuracy and safety of the intervention. The development of specialized electrodes and catheters tailored for specific anatomical sites and lesion characteristics, such as flexible catheters for cardiac arrhythmias or specialized probes for nerve ablation, further exemplifies the ongoing technological refinement in this market.
Looking ahead, the integration of Artificial Intelligence (AI) and robotic assistance is poised to be a transformative force in the RFA technology landscape. AI algorithms are being developed to analyze medical images for automated lesion detection, optimize electrode placement, and predict ablation zone dimensions, leading to more personalized and precise treatments. Robotic-assisted RFA systems offer enhanced maneuverability, tremor reduction, and the potential for remote operation, significantly improving procedural control and consistency, especially in challenging anatomical locations. Furthermore, advancements in real-time impedance monitoring, tissue characterization, and the development of non-invasive sensors are contributing to smarter and more adaptive RFA systems. The focus remains on developing devices that are not only highly effective but also minimally invasive, user-friendly, and capable of integrating seamlessly into the rapidly evolving digital operating room environment, ultimately aiming to improve patient outcomes and expand therapeutic indications.
Regional Highlights
- North America: Dominant market share due to advanced healthcare infrastructure, high prevalence of chronic diseases (cancer, cardiovascular conditions, chronic pain), favorable reimbursement policies, and significant investments in R&D and adoption of innovative medical technologies. The United States accounts for the largest share within the region, driven by strong market players and high patient awareness.
- Europe: A mature market with steady growth, characterized by an aging population, increasing incidence of chronic diseases, and a robust healthcare system. Countries like Germany, France, and the UK are key contributors, benefiting from high healthcare expenditure and the adoption of minimally invasive procedures. Emphasis on early diagnosis and treatment also boosts market expansion.
- Asia Pacific (APAC): Fastest-growing region, fueled by rapidly developing healthcare infrastructure, increasing healthcare expenditure, a large patient pool, and growing medical tourism. Countries such as China, India, Japan, and South Korea are key markets, driven by rising awareness of advanced treatments, economic growth, and government initiatives to improve healthcare access and quality.
- Latin America: An emerging market experiencing significant growth due to improving economic conditions, increasing investment in healthcare facilities, and rising prevalence of chronic diseases. Brazil and Mexico are leading the adoption of RFA devices, as healthcare systems strive to offer advanced treatment options.
- Middle East and Africa (MEA): A nascent market with considerable growth potential. Development of modern healthcare facilities, increasing medical tourism, and a rising incidence of chronic diseases, particularly in countries like UAE, Saudi Arabia, and South Africa, are driving the adoption of RFA technologies. However, challenges such as limited access to advanced healthcare and lower healthcare spending in some parts of the region remain.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Radiofrequency Ablation Devices Market.- Medtronic
- Johnson & Johnson (Ethicon)
- Boston Scientific Corporation
- Abbott Laboratories (St. Jude Medical)
- Smith & Nephew
- Hologic, Inc.
- Merit Medical Systems, Inc.
- AtriCure, Inc.
- Olympus Corporation
- AngioDynamics, Inc.
- RF Medical Co., Ltd.
- Baylis Medical Company Inc. (Boston Scientific)
- STARmed Co., Ltd.
- Inomed Medizintechnik GmbH
- Accuray Incorporated
- Brainlab AG
- Integra LifeSciences Corporation
- ConMed Corporation
- Cosman Medical, Inc.
- Diros Technology Inc.
Frequently Asked Questions
Analyze common user questions about the Radiofrequency Ablation Devices market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is radiofrequency ablation and what conditions does it treat?
Radiofrequency ablation (RFA) is a minimally invasive medical procedure that uses high-frequency electrical current to heat and destroy targeted abnormal tissues or nerve pathways. It effectively treats a variety of conditions, including malignant tumors in organs like the liver, lung, and kidney, chronic pain such as back and neuropathic pain, cardiac arrhythmias like atrial fibrillation, and uterine fibroids.
How does a radiofrequency ablation device work to destroy tissue?
RFA devices work by delivering high-frequency alternating current through an electrode (probe or catheter) inserted into the target tissue. As the electrical current passes through the tissue, it generates heat due to resistive friction, causing cellular necrosis and effectively destroying the targeted cells or denervating pain pathways without requiring extensive surgery.
What are the primary benefits of choosing radiofrequency ablation over traditional surgical options?
The primary benefits of RFA include its minimally invasive nature, leading to smaller incisions, reduced pain, and faster recovery times for patients compared to open surgical procedures. It often results in fewer complications, a lower risk of infection, and can be performed on an outpatient basis or with shorter hospital stays, offering significant advantages in patient comfort and healthcare cost efficiency.
Are there any risks or potential side effects associated with radiofrequency ablation procedures?
While generally safe, RFA procedures carry potential risks and side effects, including minor pain or bruising at the insertion site, infection, bleeding, or thermal injury to surrounding healthy tissues. In rare cases, more severe complications like nerve damage or incomplete ablation may occur. The specific risks depend on the treated area and the patient's overall health.
What is the future outlook for the Radiofrequency Ablation Devices Market?
The future outlook for the RFA Devices Market is highly positive, driven by technological advancements like AI integration and robotic assistance, expanding applications in new therapeutic areas, and increasing demand for minimally invasive treatments globally. Market growth will be particularly strong in emerging economies, with ongoing innovation aimed at enhancing precision, safety, and personalization of RFA therapies.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
