Sickle Cell Disease Treatment Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427900 | Date : Oct, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Sickle Cell Disease Treatment Market Size
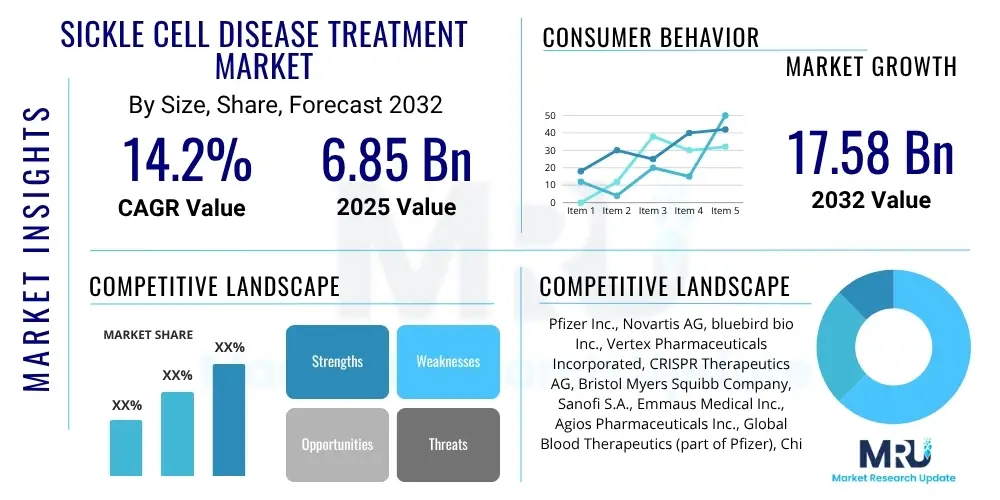
The Sickle Cell Disease Treatment Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 14.2% between 2025 and 2032. The market is estimated at USD 6.85 Billion in 2025 and is projected to reach USD 17.58 Billion by the end of the forecast period in 2032.
Sickle Cell Disease Treatment Market introduction
The Sickle Cell Disease (SCD) Treatment Market is a rapidly evolving sector dedicated to addressing the profound medical challenges posed by this inherited blood disorder. Sickle cell disease is characterized by a genetic mutation that causes red blood cells to become rigid, crescent-shaped, and unable to effectively carry oxygen throughout the body. This leads to chronic anemia, severe pain episodes known as sickle cell crises, frequent infections, and progressive organ damage, significantly reducing life expectancy and quality of life for millions globally. The market encompasses a broad range of therapeutic interventions, from conventional symptom management and supportive care to groundbreaking disease-modifying therapies and potentially curative treatments. Key product descriptions include established medications like hydroxyurea, which helps reduce the frequency of pain crises and acute chest syndrome, along with newer approved therapies such as L-glutamine, voxelotor, and crizanlizumab, each targeting specific pathways involved in the pathophysiology of SCD to alleviate symptoms and prevent complications. Furthermore, the market is on the cusp of a revolutionary shift with the emergence of advanced gene therapies and gene-editing techniques that aim to correct the underlying genetic defect.
Major applications for these treatments are focused on managing the multifaceted manifestations of SCD across various patient populations, from pediatric to adult, and are increasingly tailored to individual patient profiles and disease severity. Benefits derived from these treatments are substantial, ranging from improved blood flow and reduced hemolytic anemia to a significant decrease in the incidence and severity of vaso-occlusive crises, which are the primary cause of acute pain and organ damage in SCD patients. This leads to enhanced patient quality of life, fewer hospitalizations, and a potential extension of lifespan. The primary driving factors for the market's robust growth include the increasing global prevalence of sickle cell disease, particularly in regions like Africa, the Middle East, and South Asia, coupled with a growing awareness and improved diagnostic capabilities that lead to earlier detection and intervention. Furthermore, substantial advancements in understanding the molecular mechanisms of SCD have paved the way for innovative drug discovery and development, with significant R&D investments from pharmaceutical and biotechnology companies. Favorable regulatory designations, such as Orphan Drug status and Breakthrough Therapy designations, are also accelerating the approval process for novel treatments, underscoring the urgent unmet medical need within the SCD community and further catalyzing market expansion.
Sickle Cell Disease Treatment Market Executive Summary
The Sickle Cell Disease Treatment Market is poised for significant expansion, driven by a confluence of evolving business trends, distinct regional dynamics, and innovative segment-specific developments. From a business perspective, the market is characterized by a strong emphasis on strategic collaborations, licensing agreements, and mergers and acquisitions, as larger pharmaceutical companies seek to integrate promising new therapies developed by biotechnology firms. This consolidation reflects the high capital requirements for advanced therapy development and the strategic advantage of diversified portfolios. Moreover, a shift towards value-based healthcare models is influencing market strategies, with an increasing focus on demonstrating the long-term cost-effectiveness and improved patient outcomes of novel treatments, rather than solely relying on drug efficacy. Companies are also investing heavily in patient support programs and educational initiatives to improve adherence and access, especially for complex and high-cost gene therapies. The pipeline is robust, featuring a growing number of precision medicines and curative approaches, signalling a transformative period for SCD management.
Regionally, North America and Europe currently dominate the market due to robust healthcare infrastructures, high R&D spending, favorable reimbursement policies, and a significant patient pool with access to advanced diagnostics and treatments. However, the Asia Pacific, Latin America, and Middle East & Africa regions are projected to exhibit the highest growth rates during the forecast period. This surge is attributed to increasing disease awareness, improving healthcare access, a large undiagnosed or undertreated patient population, and rising government initiatives aimed at improving healthcare outcomes in these developing economies. For instance, countries in Sub-Saharan Africa, where SCD prevalence is highest, are witnessing growing efforts to establish better diagnostic and treatment facilities, supported by international aid and local health programs. These emerging markets represent significant untapped potential for pharmaceutical companies seeking to expand their global footprint, although they present unique challenges related to infrastructure, affordability, and regulatory landscapes.
Segment-wise, the market is witnessing a strong trend towards disease-modifying therapies (DMTs) and curative options. While conventional treatments like pain management and blood transfusions remain foundational, the emphasis has markedly shifted towards therapies that address the root cause of the disease or significantly alter its progression. Gene therapy and gene-editing technologies, though nascent, are garnering immense interest and investment, representing the frontier of SCD treatment. Within pharmaceutical modalities, oral small molecules and monoclonal antibodies are experiencing strong adoption due due to their targeted mechanisms and favorable administration profiles. The hospital pharmacy segment continues to be a primary channel for drug distribution, especially for infusion-based therapies, but specialty pharmacies are gaining prominence for dispensing high-cost, complex oral medications. Furthermore, the diagnostic segment is evolving with non-invasive and rapid screening tests, which are critical for early intervention, particularly in newborns, contributing to improved long-term outcomes and shaping treatment pathways. These trends collectively underscore a dynamic and promising future for the Sickle Cell Disease Treatment Market, driven by innovation and a growing commitment to addressing this debilitating condition.
AI Impact Analysis on Sickle Cell Disease Treatment Market
Users frequently inquire about how artificial intelligence (AI) is transforming the landscape of Sickle Cell Disease (SCD) treatment, particularly concerning its potential to accelerate drug discovery, optimize patient care, and personalize therapeutic approaches. Common questions revolve around AI's role in identifying novel drug targets, improving the efficiency of clinical trials, enabling more precise diagnostic tools, and managing the complex, lifelong symptoms of SCD. There's significant interest in whether AI can predict and prevent vaso-occlusive crises, analyze genetic data for personalized medicine, and streamline the administrative burden on healthcare providers. Users also express expectations regarding AI's capability to enhance accessibility to care in underserved regions and to lower the overall cost of treatment by making development processes more efficient. The overarching theme is one of cautious optimism, acknowledging the immense potential for AI to revolutionize SCD treatment while also considering the challenges of data privacy, algorithmic bias, and the ethical implementation of such advanced technologies in patient care.
The integration of AI into the Sickle Cell Disease treatment market is rapidly gaining momentum, offering unprecedented opportunities to address the complex challenges associated with this debilitating genetic disorder. AI-driven platforms are being leveraged across the entire drug development lifecycle, from foundational research to post-market surveillance. In the early stages, AI algorithms can analyze vast biological datasets, including genomics, proteomics, and metabolomics, to identify novel therapeutic targets and biomarkers that were previously overlooked. This capability significantly streamlines the lead identification and optimization processes, allowing researchers to quickly sift through millions of compounds to find those with the highest potential efficacy and safety profiles for SCD. Furthermore, AI contributes to repurposing existing drugs, accelerating the availability of therapies. For clinical trials, AI can optimize patient recruitment strategies by identifying suitable candidates based on predictive analytics, improving trial design, and monitoring patient responses in real-time, thereby reducing development timelines and costs.
Beyond drug discovery, AI is revolutionizing patient management and personalized medicine in SCD. Machine learning models are being developed to predict the onset of vaso-occlusive crises, a hallmark of SCD, by analyzing patient-specific data such as electronic health records, wearable sensor data, and environmental factors. This predictive capability enables proactive interventions, potentially preventing severe episodes and improving patient quality of life. AI also plays a crucial role in developing personalized treatment regimens. By analyzing an individual's genetic profile, disease severity, and response to previous therapies, AI can recommend tailored treatment plans, including optimal drug dosages and combination therapies, to maximize efficacy and minimize adverse effects. Diagnostic imaging analysis, such as MRI scans for organ damage or retinopathy, can be enhanced by AI algorithms, providing more accurate and faster interpretations for early detection and intervention. The continuous monitoring of patients through AI-powered remote sensing devices can provide real-time insights into disease progression and treatment adherence, enabling healthcare providers to intervene promptly and make data-driven decisions. This comprehensive impact positions AI as a pivotal force in transforming SCD treatment from reactive symptom management to proactive, personalized, and potentially preventive care, ultimately improving outcomes for millions of patients worldwide.
- Accelerated drug discovery and target identification through genomic and proteomic analysis.
- Optimized clinical trial design, patient stratification, and recruitment, reducing trial timelines.
- Personalized treatment planning based on individual genetic profiles and disease progression.
- Predictive analytics for vaso-occlusive crises, enabling proactive intervention and management.
- Enhanced diagnostic accuracy in imaging and laboratory analysis for early disease detection and monitoring.
- Development of smart monitoring systems for real-time patient data collection and remote care.
- Identification of drug repurposing opportunities for existing medications to treat SCD.
- Facilitation of real-world evidence generation for treatment effectiveness and safety.
- Streamlining of administrative tasks and resource allocation within healthcare systems for SCD patients.
DRO & Impact Forces Of Sickle Cell Disease Treatment Market
The Sickle Cell Disease Treatment Market is significantly influenced by a dynamic interplay of drivers, restraints, and opportunities, collectively forming the impact forces that shape its trajectory. A primary driver is the increasing global prevalence of SCD, particularly in high-burden regions like Sub-Saharan Africa, South Asia, and the Middle East, leading to an expanding patient pool that requires effective therapeutic interventions. Coupled with this, a growing awareness of the disease, improved diagnostic capabilities, and robust newborn screening programs are contributing to earlier diagnosis and initiation of treatment, thereby increasing market demand. Furthermore, the substantial unmet medical needs in SCD, where many patients still experience severe pain, organ damage, and reduced life expectancy despite existing therapies, continue to fuel intense research and development efforts. This has resulted in a vibrant pipeline of novel disease-modifying and potentially curative therapies, including gene therapies, gene-editing techniques, and innovative small molecules, which are receiving accelerated regulatory approvals and favorable designations like Orphan Drug status, pushing market growth forward.
However, several significant restraints challenge the market's full potential. The high cost associated with developing and commercializing advanced therapies, particularly gene therapies, is a major barrier to widespread adoption, especially in low- and middle-income countries where the disease burden is highest. This high cost often translates into limited patient access due to strained healthcare budgets and inadequate reimbursement policies. Diagnostic challenges, particularly in resource-limited settings, can delay diagnosis and treatment initiation, leading to advanced disease progression before intervention. The complex administration of some advanced therapies, requiring specialized medical centers and highly trained personnel, further restricts their accessibility. Moreover, the lack of comprehensive patient registries and robust real-world data collection in many regions hinders a complete understanding of disease epidemiology and treatment outcomes, impacting market forecasting and strategic planning. The potential for side effects and long-term safety concerns associated with novel, more aggressive therapies also necessitates cautious post-market surveillance and could influence adoption rates.
Amidst these challenges, considerable opportunities exist to propel the market forward. The most significant opportunity lies in the continued advancement of curative therapies, such as gene therapy and CRISPR/Cas9 gene-editing approaches, which hold the promise of transforming SCD from a chronic, lifelong condition into a curable disease. Investments in personalized medicine, utilizing genetic profiling to tailor treatments to individual patient responses, represent another key opportunity for enhancing efficacy and reducing adverse effects. Expanding market reach into emerging economies, where a large and underserved patient population resides, through innovative pricing models, strategic partnerships, and capacity building initiatives, presents immense growth potential. Developing combination therapies that target multiple pathways involved in SCD pathophysiology could offer more comprehensive disease management and improved outcomes. Furthermore, advancements in early diagnosis, especially through non-invasive and affordable screening methods, can facilitate timely intervention and significantly improve long-term prognoses. The growing role of digital health solutions, including telemedicine and AI-powered remote monitoring, offers new avenues for improving patient access, adherence, and overall disease management, particularly in geographically dispersed or resource-constrained settings. These drivers, restraints, and opportunities collectively shape a dynamic and complex market environment for Sickle Cell Disease treatment, necessitating strategic innovation and collaborative efforts to address the global burden of this disease.
Segmentation Analysis
The Sickle Cell Disease Treatment Market is intricately segmented across various dimensions, including drug type, mechanism of action, route of administration, end-user, and geography, each providing a granular view of the market's composition and growth dynamics. This comprehensive segmentation is crucial for understanding the diverse therapeutic approaches, the evolving landscape of pharmacological interventions, and the specific needs of different patient populations and healthcare settings. The market's segmentation reflects the broad spectrum of treatment modalities available, ranging from symptomatic relief and supportive care to groundbreaking disease-modifying and curative therapies. Each segment is characterized by distinct market drivers, competitive landscapes, and regulatory considerations, influencing adoption rates and market share distribution. Analyzing these segments helps stakeholders identify key growth areas, understand market preferences, and tailor development and commercialization strategies effectively. The continuous introduction of novel drugs and advanced therapeutic approaches further enriches this segmentation, leading to a dynamic market structure.
By drug type, the market includes established medications like hydroxyurea, which has been a cornerstone of SCD management for decades, alongside a new generation of targeted therapeutics. These newer drugs often fall under specific mechanisms of action, such as L-glutamine (Endari), which targets oxidative stress; voxelotor (Oxbryta), an oral hemoglobin polymerization inhibitor; and crizanlizumab (Adakveo), a monoclonal antibody that inhibits P-selectin, thereby reducing vaso-occlusive crises. The emergence of gene therapies and gene-editing technologies, though currently limited in availability, represents a distinct and highly impactful segment within the market, offering potentially curative solutions. The route of administration segmentation typically divides treatments into oral, injectable/infusion, and others, with oral therapies gaining preference due to convenience and ease of use, where applicable. The end-user segment primarily includes hospitals, specialty clinics, and homecare settings, reflecting where patients receive treatment and ongoing care. Geographically, the market is segmented into major regions such as North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa, each presenting unique epidemiological profiles, healthcare infrastructure, and market penetration levels. This detailed segmentation allows for a nuanced understanding of market trends, competitive positioning, and future growth opportunities across the Sickle Cell Disease treatment landscape.
- By Drug Type:
- Hydroxyurea
- L-glutamine (Endari)
- Voxelotor (Oxbryta)
- Crizanlizumab (Adakveo)
- Gene Therapies (e.g., Exagamglogene autotemcel, Lovotibeglogene autotemcel)
- Others (e.g., pain management drugs, iron chelating agents, antibiotics, anti-inflammatory drugs)
- By Mechanism of Action:
- Hemoglobin Polymerization Inhibitors
- P-selectin Inhibitors
- L-glutamine Analogs
- Gene Modulators/Gene Editing
- Others (e.g., anti-inflammatory, antioxidant, immunomodulatory)
- By Route of Administration:
- Oral
- Injectable/Infusion
- Others
- By End-User:
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Homecare Settings
- By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Specialty Pharmacies
Value Chain Analysis For Sickle Cell Disease Treatment Market
The value chain for the Sickle Cell Disease (SCD) Treatment Market is a complex network involving multiple stakeholders, from initial research and development to final patient delivery, characterized by specialized processes and significant regulatory oversight. The upstream analysis begins with extensive R&D activities conducted by pharmaceutical and biotechnology companies, academic institutions, and contract research organizations (CROs). This phase involves target identification, drug discovery, preclinical testing, and rigorous clinical trials (Phases I, II, III). Key activities include genetic research to understand SCD pathophysiology, molecular biology for drug design, and biomarker identification. Raw material suppliers providing active pharmaceutical ingredients (APIs), excipients, and specialized laboratory reagents are also crucial upstream contributors. The intellectual property generated during this phase, protected by patents, forms the foundation of competitive advantage in the market. The high cost and lengthy timelines associated with R&D, particularly for novel gene therapies, underscore the significant investment required at this stage.
Moving downstream, once therapies receive regulatory approval from bodies like the FDA, EMA, or PMDA, the focus shifts to manufacturing, distribution, and commercialization. Manufacturing involves large-scale production of the approved drugs, often requiring specialized facilities for biologics and cell-based therapies, ensuring quality control and adherence to Good Manufacturing Practices (GMP). Distribution channels are critical for getting treatments to patients. These include direct sales to hospitals and specialty clinics, as well as indirect channels involving wholesalers and distributors who manage inventory, logistics, and supply chain integrity. For high-value, complex therapies like gene therapies, a highly controlled and often direct-to-hospital distribution model is common to ensure proper handling and administration. Retail pharmacies and increasingly, specialty pharmacies, play a vital role in dispensing oral medications, with online pharmacies emerging as a channel for certain segments, particularly for chronic medication refills.
The final stages of the value chain involve healthcare providers, including hematologists, nurses, and specialized medical staff, who prescribe and administer treatments, as well as patient support programs that ensure adherence and address side effects. Direct channels involve pharmaceutical companies engaging directly with healthcare providers and patient advocacy groups to educate about new therapies and offer support services. Indirect channels encompass broader public health initiatives, payer negotiations, and reimbursement strategies with government health agencies and private insurance companies, which determine patient access and affordability. Post-market surveillance and pharmacovigilance are continuous activities to monitor drug safety and efficacy in real-world settings. Each stage adds value and involves distinct cost structures, profit margins, and regulatory considerations, emphasizing the collaborative and highly regulated nature of the SCD treatment market's value chain. Efficient coordination across these stages is essential for bringing effective and accessible treatments to patients globally.
Sickle Cell Disease Treatment Market Potential Customers
The potential customers for the Sickle Cell Disease Treatment Market primarily consist of individuals diagnosed with sickle cell disease and their caregivers, who actively seek therapies to manage symptoms, prevent complications, and improve their quality of life. This core group includes both pediatric and adult patients across various stages of the disease, from those newly diagnosed through newborn screening programs to adults living with chronic complications. Given the lifelong nature of SCD, patients often require continuous and evolving treatment regimens, making them consistent consumers of therapies. Beyond individual patients, the healthcare institutions that treat them are crucial direct customers. These include large hospital systems, specialized hematology clinics, academic medical centers, and outpatient clinics that diagnose, manage, and administer SCD treatments. These institutions are responsible for procuring and stocking a range of medications, from established treatments like hydroxyurea to advanced gene therapies, based on their patient population needs and clinical guidelines. The decisions made by these institutions significantly influence market demand and product adoption.
Furthermore, government health agencies and private insurance providers represent another critical segment of potential customers, albeit indirectly. They act as payers and decision-makers regarding reimbursement policies, formulary inclusions, and healthcare access initiatives. Their policies directly impact the affordability and availability of SCD treatments for patients, particularly for high-cost novel therapies. Public health programs, especially in countries with high SCD prevalence, are increasingly investing in screening, prevention, and treatment services, making them significant buyers of diagnostic tools and cost-effective medications. Patient advocacy groups also play a crucial role in influencing demand by raising awareness, supporting research, and advocating for better access to care and novel treatments. Researchers and academic institutions also serve as indirect customers, utilizing market products for further clinical studies and development. The diverse spectrum of potential customers, ranging from individual patients to large institutional purchasers and payers, highlights the multi-layered market dynamics and the need for comprehensive strategies that address the needs and constraints of each customer segment to ensure broad access to effective SCD treatments.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 6.85 Billion |
| Market Forecast in 2032 | USD 17.58 Billion |
| Growth Rate | 14.2% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Pfizer Inc., Novartis AG, bluebird bio Inc., Vertex Pharmaceuticals Incorporated, CRISPR Therapeutics AG, Bristol Myers Squibb Company, Sanofi S.A., Emmaus Medical Inc., Agios Pharmaceuticals Inc., Global Blood Therapeutics (part of Pfizer), Chiesi Global Rare Diseases, GlycoMimetics Inc., Magenta Therapeutics Inc., Editas Medicine Inc., Sangamo Therapeutics Inc., Alnylam Pharmaceuticals Inc., Roche Holding AG, Ironwood Pharmaceuticals Inc., Acceleron Pharma Inc. (acquired by Merck), Forma Therapeutics Holdings Inc. (acquired by Novo Nordisk). |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Sickle Cell Disease Treatment Market Key Technology Landscape
The Sickle Cell Disease Treatment Market is undergoing a profound transformation driven by rapid advancements in biotechnology and genetic engineering, which form the core of its key technology landscape. Historically, treatment largely centered on symptomatic management with drugs like hydroxyurea, which works by increasing fetal hemoglobin levels and reducing red blood cell sickling. While effective for many, it does not offer a cure. The current technological evolution is shifting towards disease-modifying therapies (DMTs) that target specific pathological mechanisms of SCD. Small molecule inhibitors, such as voxelotor, which works by inhibiting hemoglobin polymerization, represent a significant stride. Similarly, monoclonal antibodies like crizanlizumab, designed to block P-selectin and reduce cell adhesion, exemplify targeted biological therapies that address the underlying vascular complications. These technologies leverage advanced drug discovery platforms, including high-throughput screening and rational drug design, to identify and optimize compounds with precise biological activity.
The most revolutionary technological advancements in the SCD treatment market are rooted in gene therapy and gene-editing technologies. Gene therapy involves introducing a functional copy of the beta-globin gene into a patient's hematopoietic stem cells ex vivo, correcting the genetic defect and enabling the production of healthy hemoglobin. This complex process requires sophisticated viral vectors (e.g., lentiviral vectors) for efficient gene delivery and specialized manufacturing facilities for cell processing. Following autologous stem cell transplantation, these modified cells then produce normal red blood cells, potentially offering a functional cure. Expanding upon this, gene-editing technologies like CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats) represent an even more precise approach. CRISPR allows for targeted modification of the patient’s own DNA to either directly correct the sickle cell mutation or to reactivate the production of fetal hemoglobin (HbF) by editing regulatory regions of the gamma-globin gene. These technologies demand extraordinary precision, high safety profiles to prevent off-target edits, and robust delivery mechanisms to ensure effective cellular uptake and integration.
Further technological innovations impacting the market include advanced diagnostic tools that leverage genomics and proteomics for early and accurate diagnosis, as well as for identifying prognostic biomarkers. Digital health platforms and artificial intelligence (AI) are also emerging as crucial technologies. AI algorithms are employed in drug discovery for target identification and lead optimization, in clinical trial design for patient stratification, and in personalized medicine for predicting disease progression and optimizing treatment regimens based on an individual's genetic profile and clinical data. Wearable sensors and remote monitoring technologies provide real-time patient data, enabling proactive management and improving adherence. These technological advancements collectively promise to shift the paradigm of SCD treatment from merely managing symptoms to providing targeted, personalized, and potentially curative interventions, thereby significantly improving patient outcomes and reducing the long-term burden of the disease. The continuous integration of these cutting-edge technologies is crucial for addressing the unmet needs of the global SCD patient population and driving future market growth.
Regional Highlights
- North America: This region consistently leads the Sickle Cell Disease Treatment Market, primarily driven by a robust healthcare infrastructure, high research and development expenditures, the presence of major pharmaceutical and biotechnology companies, and favorable reimbursement policies. The United States, in particular, has seen the earliest approvals and highest adoption rates for novel therapies, including gene therapies, due to strong regulatory support and significant patient advocacy. Canada also contributes to market growth with increasing awareness and access to specialized care.
- Europe: Europe represents a significant market share, characterized by increasing efforts to improve diagnostic and treatment access, particularly in countries with higher immigrant populations from endemic regions. Key countries like the UK, France, Germany, and Italy are actively involved in clinical trials and the adoption of new treatments. The European Medicines Agency (EMA) plays a crucial role in regulating and approving therapies, fostering innovation while addressing regional health disparities.
- Asia Pacific (APAC): The APAC region is projected to experience the fastest growth, largely due to its vast and largely underserved patient population, particularly in countries like India, which has one of the highest numbers of SCD births globally. Increasing healthcare expenditure, improving diagnostic capabilities, and growing awareness campaigns are key drivers. While access to advanced therapies remains a challenge, strategic partnerships and government initiatives are working towards expanding treatment availability.
- Latin America: This region, including countries like Brazil and Colombia, shows significant market potential, driven by a considerable patient population and ongoing efforts to enhance healthcare access and public health awareness regarding SCD. Challenges include economic disparities and fragmented healthcare systems, but governmental focus on rare diseases and increasing investment in healthcare infrastructure are paving the way for market expansion.
- Middle East and Africa (MEA): The MEA region holds a critical position in the SCD market due to the highest global prevalence of the disease, especially in Sub-Saharan Africa. While historically constrained by limited resources and infrastructure, growing international aid, national health programs, and increasing investment in healthcare facilities are slowly improving diagnostic and treatment access. The introduction of cost-effective and accessible therapies is paramount for this region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Sickle Cell Disease Treatment Market.- Pfizer Inc.
- Novartis AG
- bluebird bio Inc.
- Vertex Pharmaceuticals Incorporated
- CRISPR Therapeutics AG
- Bristol Myers Squibb Company
- Sanofi S.A.
- Emmaus Medical Inc.
- Agios Pharmaceuticals Inc.
- Chiesi Global Rare Diseases
- GlycoMimetics Inc.
- Magenta Therapeutics Inc.
- Editas Medicine Inc.
- Sangamo Therapeutics Inc.
- Alnylam Pharmaceuticals Inc.
- Roche Holding AG
- Ironwood Pharmaceuticals Inc.
- Gilead Sciences Inc.
- CSL Behring
- Moderna Inc.
Frequently Asked Questions
Analyze common user questions about the Sickle Cell Disease Treatment market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the newest treatments available for Sickle Cell Disease?
The newest treatments for Sickle Cell Disease include disease-modifying therapies like voxelotor (Oxbryta), crizanlizumab (Adakveo), and L-glutamine (Endari), which target specific aspects of the disease. Additionally, groundbreaking gene therapies like exagamglogene autotemcel (Casgevy) and lovotibeglogene autotemcel (Lyfgenia) have recently received regulatory approval, offering potentially curative options for eligible patients.
How effective are gene therapies for Sickle Cell Disease, and what are their limitations?
Gene therapies for SCD show high promise for long-term correction of the genetic defect, potentially offering a functional cure and eliminating symptoms. Early clinical data indicate significant reductions in vaso-occlusive crises and transfusion dependence. Limitations include the complex and intensive nature of the treatment, requiring bone marrow conditioning and specialized centers, high costs, and potential long-term safety concerns that are still being monitored.
What is the projected growth of the Sickle Cell Disease Treatment Market?
The Sickle Cell Disease Treatment Market is projected for substantial growth, estimated to reach USD 17.58 Billion by 2032 from USD 6.85 Billion in 2025, exhibiting a robust Compound Annual Growth Rate (CAGR) of 14.2% during the forecast period. This growth is driven by increasing prevalence, pipeline innovations, and improved access to therapies.
Which regions are leading in Sickle Cell Disease treatment adoption and innovation?
North America, particularly the United States, currently leads in SCD treatment adoption and innovation due to advanced healthcare infrastructure, significant R&D investment, and favorable reimbursement policies. Europe also holds a strong position. However, the Asia Pacific region is anticipated to demonstrate the fastest growth due to a large patient population and improving healthcare access.
What are the primary challenges hindering the widespread adoption of Sickle Cell Disease treatments?
Key challenges include the high cost of novel and advanced therapies, which limits patient access, especially in resource-constrained regions. Diagnostic challenges, complex administration requirements for some treatments, and the need for specialized medical infrastructure also act as significant restraints, alongside varying reimbursement policies and ethical considerations for gene-editing technologies.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
