Sirolimus Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427321 | Date : Oct, 2025 | Pages : 239 | Region : Global | Publisher : MRU
Sirolimus Market Size
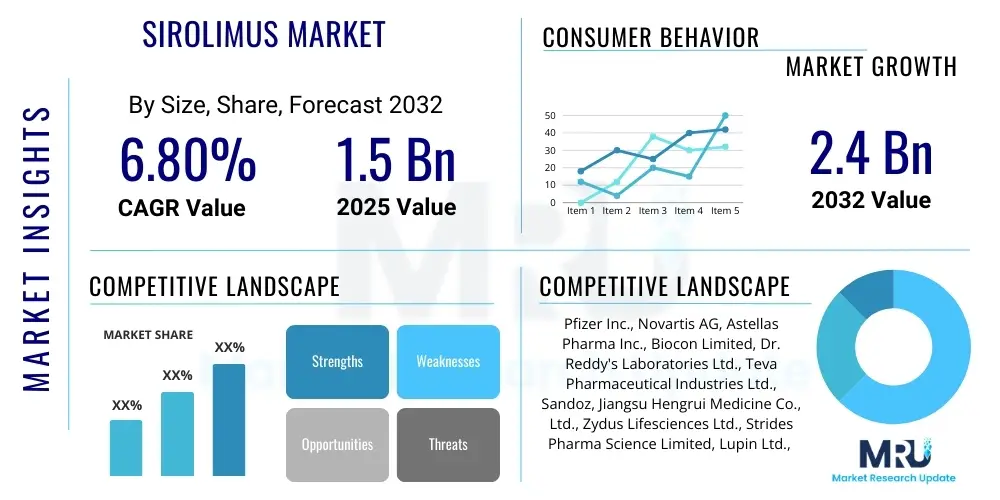
The Sirolimus Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 1.5 billion in 2025 and is projected to reach USD 2.4 billion by the end of the forecast period in 2032. This growth is primarily driven by increasing organ transplant procedures globally, the expanding therapeutic applications of sirolimus beyond its traditional uses, and continuous advancements in its formulation and delivery. The markets upward trajectory is also supported by a rising prevalence of chronic diseases necessitating transplant interventions and the growing acceptance of sirolimus as a crucial immunosuppressant.
Sirolimus Market introduction
Sirolimus, also known as rapamycin, is a macrolide compound with potent immunosuppressive properties, primarily functioning as an inhibitor of the mammalian target of rapamycin (mTOR) pathway. This unique mechanism of action distinguishes it from other immunosuppressants by preventing T-lymphocyte activation and proliferation in response to antigenic and cytokine stimulation, thereby playing a critical role in preventing organ transplant rejection. Initially discovered in the soil bacterium *Streptomyces hygroscopicus*, its therapeutic potential was quickly recognized for its ability to modulate immune responses without the nephrotoxic effects often associated with calcineurin inhibitors, making it a valuable agent in post-transplant care. The product’s versatility extends beyond immunosuppression, with a growing number of major applications emerging in various medical fields.
Major applications of sirolimus include its established use in the prophylaxis of organ rejection in kidney transplant recipients, and increasingly in heart and liver transplants. Furthermore, sirolimus has gained approval for the treatment of lymphangioleiomyomatosis (LAM), a rare progressive lung disease, and for certain types of cancer, particularly in specific renal cell carcinoma cases, owing to its antiproliferative effects. The benefits of sirolimus are multifaceted, encompassing its efficacy in preventing acute and chronic rejection, its potential to reduce the incidence of post-transplant malignancies, and its role in steroid-sparing regimens, which can significantly improve patient quality of life by mitigating steroid-related side effects. The driving factors for market growth include the rising global incidence of organ failure, a growing elderly population prone to chronic diseases, and continuous research into new indications and improved formulations, which are expanding its therapeutic utility and market reach.
Sirolimus Market Executive Summary
The Sirolimus Market is experiencing robust growth, propelled by several key business, regional, and segment trends. Business trends highlight a significant push towards generic drug development, which while increasing market accessibility, also intensifies competitive pressures for innovator companies. Strategic alliances, mergers, and acquisitions are common as companies seek to consolidate market share and expand their product portfolios to include new formulations or combination therapies. Furthermore, substantial investments in research and development are ongoing to explore novel drug delivery systems, such as nanoformulations and topical applications, aiming to enhance efficacy, reduce systemic side effects, and improve patient adherence. The focus on value-based healthcare models also influences market dynamics, pushing manufacturers to demonstrate superior clinical outcomes and cost-effectiveness.
From a regional perspective, North America and Europe continue to dominate the Sirolimus market due to well-established healthcare infrastructures, high rates of organ transplantation, and advanced diagnostic capabilities. However, the Asia Pacific region is rapidly emerging as a high-growth market, driven by improving economic conditions, increasing healthcare expenditure, a growing prevalence of chronic diseases leading to organ failure, and the expansion of medical tourism. Latin America and the Middle East & Africa also show promising growth potential as healthcare systems evolve and access to advanced medical treatments improves. Regional market penetration is significantly influenced by regulatory frameworks, healthcare policies, and the availability of skilled medical professionals, which vary considerably across different geographies.
Segmentation trends reveal that the organ transplant segment remains the largest application area for sirolimus, reflecting its critical role in preventing rejection. However, there is a notable expansion in other therapeutic segments, particularly in oncology for specific cancer types like renal cell carcinoma and neuroendocrine tumors, and in dermatology for conditions such as vascular anomalies and tuberous sclerosis complex. The market is also seeing diversification in product forms, with oral solutions and tablets being predominant, while topical formulations are gaining traction for dermatological applications. This diversification of applications and forms contributes to the overall resilience and expansion of the Sirolimus market, attracting a broader patient base and driving innovation in drug delivery and therapeutic targeting.
AI Impact Analysis on Sirolimus Market
Common user questions regarding the impact of AI on the Sirolimus Market often revolve around optimizing drug efficacy, minimizing adverse effects, and discovering new therapeutic applications. Users frequently inquire about how AI can personalize Sirolimus dosing regimes, considering individual patient metabolisms and genetic profiles, to achieve optimal immunosuppression with reduced toxicity. Concerns are also raised about AIs role in predicting potential drug-drug interactions, especially in complex post-transplant multi-drug regimens, and its capacity to identify patient subgroups most likely to benefit from Sirolimus therapy or develop specific side effects. The potential for AI to accelerate the discovery of novel mTOR inhibitors or identify new indications for Sirolimus through large-scale data analysis of clinical trials and real-world evidence is another key area of interest, reflecting a forward-looking perspective on drug development and patient management.
The integration of Artificial Intelligence (AI) and Machine Learning (ML) is poised to revolutionize several facets of the Sirolimus market, enhancing its therapeutic potential and operational efficiency. AI algorithms can analyze vast datasets of patient information, including genetic markers, pharmacokinetic profiles, and clinical outcomes, to develop highly personalized dosing strategies. This precision medicine approach can optimize Sirolimus blood levels for each individual, thereby maximizing efficacy in preventing organ rejection while simultaneously minimizing the risk of adverse events such as nephrotoxicity, hyperlipidemia, and myelosuppression. By moving beyond traditional population-based dosing, AI offers the promise of superior patient care and improved long-term graft survival rates, fundamentally transforming how Sirolimus is administered and monitored in clinical practice, leading to more predictable and safer therapeutic outcomes for transplant recipients.
- Personalized Dosing: AI algorithms can analyze individual patient pharmacogenomics and clinical data to optimize Sirolimus dosage, reducing toxicity and improving efficacy.
- Drug Discovery & Repurposing: AI can rapidly screen molecular databases and analyze existing clinical data to identify new potential indications for Sirolimus or discover novel mTOR inhibitors.
- Predictive Analytics: AI models can forecast patient responses to Sirolimus, predict the likelihood of transplant rejection, or identify patients at higher risk of developing specific side effects.
- Patient Monitoring & Adherence: Wearable sensors and AI-powered platforms can monitor patient vitals and drug adherence in real-time, providing timely interventions and improving treatment outcomes.
- Clinical Trial Optimization: AI can streamline patient selection for clinical trials, accelerate data analysis, and identify optimal trial designs, speeding up the development of new Sirolimus formulations or combinations.
- Adverse Event Management: AI can analyze patterns in adverse event reporting to better understand Sirolimus side effect profiles and develop proactive management strategies.
DRO & Impact Forces Of Sirolimus Market
The Sirolimus market is significantly shaped by a dynamic interplay of drivers, restraints, opportunities, and broader impact forces. Key drivers include the escalating global incidence of organ failure, necessitating more transplant procedures, coupled with a growing geriatric population that often requires sophisticated immunosuppressive regimens. The expanding research into new therapeutic indications for sirolimus, particularly in oncology, dermatology, and rare diseases, is also a substantial growth catalyst. Moreover, advancements in drug delivery systems and formulations aimed at improving bioavailability and reducing side effects are enhancing its market appeal. The rising awareness and improved diagnostic capabilities for conditions like lymphangioleiomyomatosis (LAM) further contribute to increased demand, broadening the patient base for sirolimus therapy and driving its continuous market expansion.
Conversely, several restraints impede the market’s full potential. The significant side effect profile of sirolimus, including hyperlipidemia, pneumonitis, and delayed wound healing, necessitates careful patient monitoring and can limit its use in certain populations. The relatively high cost of innovator sirolimus, particularly in regions with constrained healthcare budgets, also poses a barrier, although generic versions are mitigating this to some extent. Stringent regulatory approval processes for new indications or formulations add to development costs and timelines, impacting market entry for new products. Furthermore, the complexity of drug-drug interactions, especially with other immunosuppressants or medications for co-morbidities, requires intricate clinical management, presenting a challenge for widespread adoption and patient safety.
Opportunities for growth are abundant, particularly in the development of novel formulations like topical sirolimus for dermatological conditions or extended-release oral formulations that could improve patient adherence and reduce dosing frequency. The exploration of sirolimus in combination therapies for transplant patients, aiming for synergistic effects and reduced overall drug toxicity, represents another promising avenue. Furthermore, its potential in treating a wider array of rare diseases, beyond LAM, and its antineoplastic properties in various cancers are subjects of ongoing research that could unlock significant market expansion. The impact forces influencing the market are broad, encompassing evolving regulatory landscapes, global healthcare expenditure trends, rapid technological advancements in pharmacology and patient monitoring, and prevailing economic conditions that affect healthcare access and affordability. These forces collectively dictate the pace of innovation, market access, and ultimately, the uptake of sirolimus across its diverse applications, requiring continuous strategic adaptation from market players.
Segmentation Analysis
The Sirolimus market is comprehensively segmented based on various critical parameters, providing a nuanced understanding of its intricate dynamics and growth trajectories across different applications, product forms, and distribution channels. This segmentation allows for precise market analysis, enabling stakeholders to identify key growth areas, understand competitive landscapes within specific niches, and tailor strategies to address distinct market needs. The versatility of sirolimus, stemming from its unique mechanism of action, has led to its adoption in diverse medical fields, each contributing significantly to the overall market value. By dissecting the market into these actionable segments, the report offers a granular view of where growth is concentrated and where future opportunities are most likely to emerge, guiding both product development and market entry strategies for pharmaceutical companies.
- By Application:
- Organ Transplant (Kidney, Heart, Liver, Lung, Others)
- Oncology (Renal Cell Carcinoma, Neuroendocrine Tumors, Others)
- Lymphangioleiomyomatosis (LAM)
- Dermatology (Vascular Anomalies, Tuberous Sclerosis Complex, Others)
- Other Rare Diseases
- By Product Form:
- Oral Solution
- Tablets (Standard Release, Extended Release)
- Topical Formulations
- Injectables (Under Development/Specific Use)
- By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Specialty Pharmacies
Sirolimus Market Value Chain Analysis
The Sirolimus market value chain is a complex network involving multiple stakeholders, spanning from raw material sourcing to end-user consumption. Upstream analysis focuses on the procurement of Active Pharmaceutical Ingredients (APIs) and excipients, which are critical for the manufacturing process. This stage involves specialized chemical suppliers, often operating under stringent quality controls and regulatory compliance to ensure the purity and consistency of raw materials. Research and development activities, including clinical trials for new indications or formulations, also constitute a significant upstream component, demanding substantial investment and expertise. Manufacturing processes, involving fermentation, synthesis, and purification of sirolimus, are highly complex and specialized, requiring advanced facilities and adherence to Good Manufacturing Practices (GMP) to produce the final drug substance and finished dosage forms.
Moving downstream, the value chain encompasses distribution, marketing, and sales activities that deliver sirolimus to healthcare providers and ultimately to patients. This involves a robust network of wholesalers, distributors, and pharmaceutical logistics companies responsible for warehousing, inventory management, and timely delivery of the product. Distribution channels are typically categorized into direct and indirect routes. Direct channels often involve pharmaceutical companies engaging directly with large hospital systems or transplant centers, particularly for specialized or high-volume orders. Indirect channels leverage a network of third-party distributors and wholesalers who then supply to retail pharmacies, smaller clinics, and specialty pharmacies, ensuring broader market reach and accessibility. Marketing and sales efforts are crucial at this stage, focusing on educating healthcare professionals about the drugs benefits, efficacy, and safety profiles, as well as engaging with key opinion leaders to drive adoption.
The role of direct distribution is often prominent for high-value, specialized pharmaceuticals like sirolimus, especially when targeted at specific medical institutions such as transplant centers or oncology units that have specific procurement needs and patient populations. Indirect distribution, facilitated by a broad network of wholesale and retail partners, ensures that sirolimus is available through a wider array of pharmacies for outpatient use and for patients managing chronic conditions. Both channels require careful management to ensure product integrity, regulatory compliance, and efficient delivery to the end-users. The interplay between these upstream and downstream activities, supported by effective distribution channels, is crucial for the continuous flow of sirolimus from its origin as a raw material to its final therapeutic application in patients, driving the markets efficiency and reach while maintaining high standards of quality and safety throughout the entire process.
Sirolimus Market Potential Customers
The primary potential customers and end-users of sirolimus are diverse, reflecting its expanding range of therapeutic applications across various medical specialties. Transplant centers represent a foundational customer segment, as sirolimus is a cornerstone immunosuppressant used in the prophylaxis of organ rejection following kidney, heart, liver, and lung transplants. Within these centers, nephrologists, cardiologists, hepatologists, pulmonologists, and transplant surgeons are key prescribers, alongside specialized transplant pharmacists who manage patient medication regimens. These institutions prioritize drugs that offer superior efficacy, a favorable side effect profile in complex multi-drug environments, and robust clinical evidence to support long-term graft survival, making sirolimus a vital component of their therapeutic arsenal for improving patient outcomes and quality of life post-transplant.
Beyond the transplant community, oncologists constitute a growing customer base, particularly those specializing in specific cancer types where sirolimus, as an mTOR inhibitor, demonstrates clinical utility. This includes its use in advanced renal cell carcinoma, certain neuroendocrine tumors, and other malignancies where the mTOR pathway plays a critical role in disease progression. Dermatologists are also increasingly recognizing sirolimus for its efficacy in treating conditions like vascular anomalies, including lymphatic malformations, and skin manifestations of tuberous sclerosis complex. Patients suffering from rare diseases such as lymphangioleiomyomatosis (LAM), where sirolimus is the only approved treatment, form another distinct and crucial customer segment, relying on the drug to manage their debilitating symptoms and improve lung function. The broadening indications continue to attract new specialists and patient populations to sirolimus therapy, expanding its market potential.
Therefore, the customer base extends to a wide array of healthcare providers and patients. Hospitals and specialized clinics, particularly those with departments for organ transplantation, oncology, and rare disease management, are significant institutional buyers. Retail pharmacies and increasingly, online pharmacies, cater to individual patients who require ongoing prescriptions for chronic conditions or post-transplant care. The ultimate beneficiaries are the patients themselves, who rely on sirolimus to maintain their health, prevent life-threatening complications, and manage chronic conditions effectively. As research continues to uncover new applications and as healthcare access improves globally, the spectrum of potential customers for sirolimus is expected to broaden further, driving sustained demand across a diverse range of medical settings and patient needs, underscoring its pivotal role in modern medicine.
Sirolimus Market Key Technology Landscape
The technological landscape surrounding the Sirolimus market is characterized by continuous innovation aimed at enhancing its therapeutic effectiveness, improving patient safety, and expanding its utility. A crucial area of technological advancement lies in advanced drug delivery systems. Researchers are exploring novel formulations such as nanoparticles, microemulsions, and liposomal encapsulations to improve the bioavailability of sirolimus, which historically has presented challenges due to its poor water solubility and variable absorption. These advanced systems aim to achieve more consistent drug levels, reduce the frequency of dosing, and potentially target specific tissues or organs, thereby minimizing systemic side effects and improving the therapeutic index. Such innovations are critical for optimizing patient outcomes and expanding the applicability of sirolimus in diverse patient populations, including those with compromised absorption or complex drug regimens.
Another significant technological trend involves the integration of personalized medicine approaches, particularly pharmacogenomics, into sirolimus therapy. Genetic testing can identify individual variations in drug metabolism enzymes, such as CYP3A4 and ABCB1, which play a key role in sirolimus pharmacokinetics. By understanding a patients genetic profile, clinicians can tailor initial sirolimus doses and make more informed adjustments, reducing the trial-and-error approach often associated with immunosuppressant dosing. This technology not only enhances the safety and efficacy of treatment by minimizing the risk of sub-therapeutic levels leading to rejection or supra-therapeutic levels causing toxicity, but also paves the way for a more predictive and patient-centric approach to immunosuppression. This level of precision medicine represents a transformative step in managing transplant patients and other conditions treated with sirolimus.
Furthermore, the use of digital health technologies, including remote monitoring devices and Artificial Intelligence (AI) powered analytics, is increasingly impacting the sirolimus market. Digital platforms can track patient adherence to medication, monitor vital signs, and collect real-world data on side effects and treatment outcomes. AI algorithms can then analyze this vast amount of data to identify patterns, predict potential complications, and provide decision support to clinicians, enabling proactive management of sirolimus therapy. This technological synergy allows for real-time adjustments, enhances patient engagement, and improves overall long-term care for individuals on sirolimus. These advancements, spanning from novel drug delivery to personalized genomics and digital health, collectively contribute to making sirolimus a safer, more effective, and more precisely managed therapeutic option in various medical applications, driving its sustained relevance and growth within the pharmaceutical sector.
Regional Highlights
- North America: This region holds a significant share of the Sirolimus market, driven by high healthcare expenditure, well-developed healthcare infrastructure, a large number of organ transplant procedures, and extensive research and development activities. The presence of key pharmaceutical players and a high prevalence of chronic diseases contribute to robust market demand. Advanced diagnostic capabilities and patient awareness further support market growth.
- Europe: Europe represents another major market for Sirolimus, characterized by strong regulatory frameworks, a high standard of medical care, and a substantial number of organ transplants, particularly in Western European countries. Government initiatives promoting organ donation and advanced healthcare reimbursement policies also facilitate market expansion. The region also benefits from a strong pharmaceutical research base.
- Asia Pacific: Expected to be the fastest-growing region, the Asia Pacific market is fueled by improving healthcare infrastructure, increasing disposable incomes, a large patient pool with rising incidences of chronic diseases, and growing awareness of advanced medical treatments. Countries like China, India, and Japan are investing heavily in healthcare, leading to an increase in transplant procedures and broader adoption of immunosuppressants. Medical tourism also plays a role in driving demand.
- Latin America: This region demonstrates a steady growth trajectory, supported by increasing healthcare investments, a rising number of organ transplant programs, and expanding access to specialized medical care. Economic development and government initiatives aimed at improving public health contribute to the gradual expansion of the Sirolimus market here. Local manufacturing capabilities are also slowly emerging.
- Middle East & Africa: The market in the Middle East & Africa is experiencing nascent but growing demand, driven by increasing healthcare expenditure, a rising prevalence of chronic kidney disease and other organ failures, and the development of specialized medical centers. Efforts to modernize healthcare systems and improve access to advanced therapies are key factors influencing market growth in this diverse region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Sirolimus Market.- Pfizer Inc.
- Novartis AG
- Astellas Pharma Inc.
- Biocon Limited
- Dr. Reddys Laboratories Ltd.
- Teva Pharmaceutical Industries Ltd.
- Sandoz (Novartis division)
- Jiangsu Hengrui Medicine Co., Ltd.
- Zydus Lifesciences Ltd.
- Strides Pharma Science Limited
- Lupin Ltd.
- Accord Healthcare (Intas Pharmaceuticals Ltd.)
- Sun Pharmaceutical Industries Ltd.
Frequently Asked Questions
What is Sirolimus primarily used for in medicine?
Sirolimus is primarily used as an immunosuppressant to prevent organ rejection in patients who have received kidney, heart, or other organ transplants. It is also approved for treating lymphangioleiomyomatosis (LAM), a rare progressive lung disease, and has applications in certain oncology and dermatological conditions. Its unique mechanism of action as an mTOR inhibitor makes it effective in modulating the immune response and inhibiting cell proliferation.
How does Sirolimus work to prevent organ rejection?
Sirolimus works by inhibiting the mammalian target of rapamycin (mTOR), a crucial protein involved in cell growth, proliferation, and immune function. By binding to the immunophilin FKBP12, it forms a complex that inhibits mTOR, thereby preventing T-lymphocyte activation and proliferation. This action reduces the immune systems ability to attack and reject the transplanted organ, providing effective immunosuppression distinct from calcineurin inhibitors, often used in combination for enhanced efficacy.
What are the common side effects associated with Sirolimus?
Common side effects of Sirolimus can include hyperlipidemia (high cholesterol and triglycerides), stomatitis (mouth sores), skin rash, diarrhea, headache, and joint pain. More serious side effects can involve myelosuppression (low blood cell counts), pneumonitis (inflammation of the lungs), delayed wound healing, and an increased risk of infections and certain malignancies. Regular monitoring of blood levels and patient health is essential to manage these potential adverse events effectively.
Is Sirolimus available as a generic medication?
Yes, Sirolimus is available as a generic medication from several manufacturers. The availability of generic versions has significantly increased market accessibility and affordability for patients, particularly in regions with budget constraints, making it a more viable option for long-term immunosuppressive therapy. Generic versions must meet stringent bioequivalence standards to ensure they are therapeutically equivalent to the innovator product, offering comparable efficacy and safety profiles.
How is Sirolimus dosing typically determined and monitored?
Sirolimus dosing is highly individualized and typically determined based on the patients weight, age, type of transplant, and co-administered medications. Therapeutic drug monitoring (TDM) is crucial, involving regular measurement of Sirolimus blood concentrations to maintain levels within a narrow therapeutic window. This ensures optimal immunosuppression while minimizing toxicity. Adjustments are frequently made based on clinical response, side effects, and drug-drug interactions, often guided by pharmacokinetic principles to achieve personalized and effective treatment outcomes.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
