Sleep Apnea Implants Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 430928 | Date : Nov, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Sleep Apnea Implants Market Size
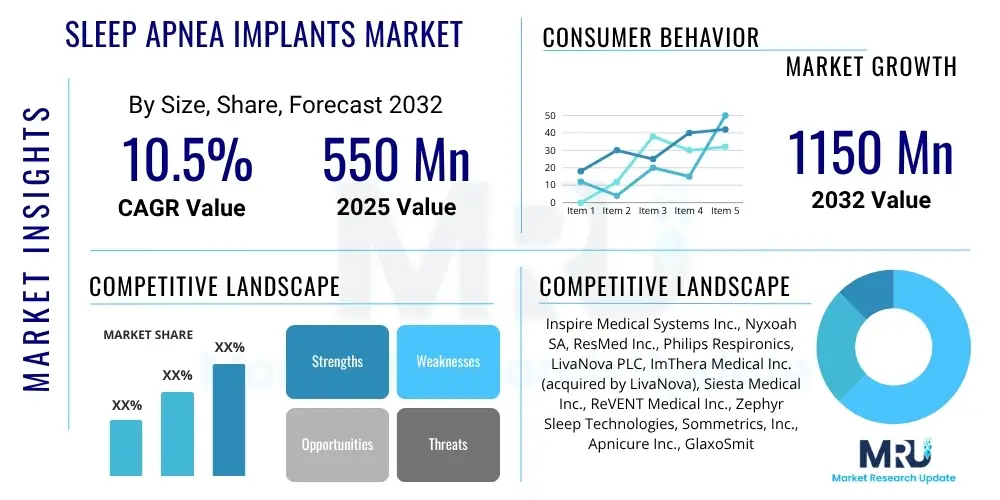
The Sleep Apnea Implants Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 10.5% between 2025 and 2032. The market is estimated at USD 550 Million in 2025 and is projected to reach USD 1150 Million by the end of the forecast period in 2032.
Sleep Apnea Implants Market introduction
The Sleep Apnea Implants Market encompasses medical devices designed to treat Obstructive Sleep Apnea (OSA), a chronic condition characterized by repeated episodes of upper airway collapse during sleep. These innovative solutions offer an alternative for patients who cannot tolerate or achieve adequate results with traditional therapies such as Continuous Positive Airway Pressure (CPAP) therapy. The primary objective of these implants is to maintain airway patency, thereby improving sleep quality, reducing associated health risks, and enhancing overall patient quality of life.
Products within this market typically include hypoglossal nerve stimulation systems and palatal implants, each working through different mechanisms to prevent airway obstruction. Hypoglossal nerve stimulators, for instance, involve a small, implantable device that monitors breathing and stimulates the hypoglossal nerve to move the tongue forward, opening the airway during sleep. Palatal implants, on the other hand, aim to stiffen the soft palate, reducing its vibration and collapse.
Major applications of sleep apnea implants are primarily focused on individuals diagnosed with moderate to severe OSA who have failed or are intolerant to CPAP therapy. The benefits extend beyond symptom reduction, encompassing significant improvements in daytime alertness, cardiovascular health, and a decrease in the risk of comorbidities linked to untreated OSA. Key driving factors for market growth include the escalating global prevalence of OSA, increasing awareness among both patients and clinicians, ongoing technological advancements leading to more effective and less invasive devices, and the persistent challenge of CPAP non-adherence among a substantial patient population.
Sleep Apnea Implants Market Executive Summary
The Sleep Apnea Implants Market is currently experiencing robust growth, driven by an aging global population, rising obesity rates contributing to higher OSA prevalence, and a growing unmet need for effective treatment alternatives for CPAP-intolerant patients. Business trends indicate a focus on strategic partnerships, mergers and acquisitions aimed at expanding product portfolios and geographical reach, alongside significant investments in research and development to introduce next-generation devices with enhanced efficacy, smaller footprints, and improved patient comfort. Companies are increasingly exploring comprehensive patient management platforms that integrate diagnostic tools, treatment devices, and remote monitoring solutions to offer holistic care.
Regional trends highlight North America as the dominant market, attributable to a well-established healthcare infrastructure, high awareness levels, and favorable reimbursement policies. Europe is also a significant contributor, showing steady growth with increasing adoption of advanced therapies and a strong emphasis on clinical research. The Asia Pacific region is anticipated to exhibit the fastest growth over the forecast period, propelled by a large undiagnosed patient population, improving healthcare access, rising disposable incomes, and increasing healthcare expenditure in developing economies. Latin America, the Middle East, and Africa are emerging markets, presenting long-term opportunities as healthcare systems evolve and awareness campaigns gain traction.
Segment trends within the market demonstrate a clear preference for hypoglossal nerve stimulation devices due to their clinical effectiveness and widespread acceptance among patients and clinicians. The end-user segment sees hospitals and specialized sleep centers as primary consumers, though ambulatory surgical centers are gaining prominence due to the minimally invasive nature of implant procedures. Efforts are also being made to broaden the indication for these implants to a wider range of OSA severities and patient profiles, thereby expanding the overall market potential and addressing a larger segment of the afflicted population seeking lasting relief from sleep apnea symptoms.
AI Impact Analysis on Sleep Apnea Implants Market
Users frequently inquire about how Artificial Intelligence (AI) can revolutionize the diagnosis, treatment, and ongoing management of sleep apnea, particularly concerning implantable devices. There is keen interest in AI's potential to enhance diagnostic accuracy, personalize treatment selection for optimal patient outcomes, and optimize the performance of existing and future sleep apnea implants. Key themes emerging from these inquiries include the expectation of AI-powered diagnostic tools reducing the burden of traditional sleep studies, the promise of predictive analytics to identify ideal candidates for implant therapy, and the integration of AI into implant devices for adaptive stimulation and remote monitoring capabilities. Concerns often revolve around data privacy, regulatory hurdles for AI-driven medical devices, and the need for robust clinical validation of AI's effectiveness in this complex medical field.
- AI for enhanced diagnosis and patient stratification, potentially leading to earlier and more accurate OSA detection.
- Predictive analytics leveraging AI to identify optimal candidates for sleep apnea implant therapy, improving treatment success rates.
- Personalized implant settings and adaptive stimulation algorithms, allowing devices to adjust therapy in real-time based on sleep patterns.
- Integration of AI into remote monitoring systems for proactive management, early detection of issues, and improved patient compliance.
- Development of AI-powered surgical planning tools to enhance the precision and safety of implant procedures.
- AI-driven analysis of post-implantation data to refine treatment protocols and improve long-term efficacy.
- Optimized R&D processes through AI simulations, accelerating the development of next-generation implant technologies.
DRO & Impact Forces Of Sleep Apnea Implants Market
The Sleep Apnea Implants Market is significantly shaped by a confluence of driving factors, notable restraints, and compelling opportunities, all subject to various impact forces. A primary driver is the escalating global prevalence of Obstructive Sleep Apnea, which continues to rise due to increasing rates of obesity and an aging population, creating a larger pool of patients requiring effective treatment. Coupled with this, the high rate of non-adherence and intolerance to CPAP therapy, the current gold standard, pushes a substantial number of patients to seek alternative solutions, making implants an increasingly attractive option. Furthermore, continuous advancements in implant technology, leading to more sophisticated, miniaturized, and patient-friendly devices, contribute significantly to market expansion. Favorable reimbursement policies in key developed markets are also critical in boosting patient access and adoption of these often expensive treatments.
However, the market faces several notable restraints. The high cost associated with sleep apnea implants, including the device itself, the surgical procedure, and follow-up care, remains a significant barrier for many patients, particularly in regions with less comprehensive healthcare coverage. Additionally, as with any surgical procedure, there are inherent risks of complications, which can deter potential candidates. Awareness and acceptance of these relatively newer therapies are still evolving in certain geographical areas and among some healthcare professionals, necessitating further educational efforts. Moreover, the need for specialized training for surgeons and sleep specialists to perform these procedures and manage patients effectively can limit widespread adoption.
Despite these challenges, substantial opportunities exist for market growth. Emerging markets, particularly in Asia Pacific and Latin America, represent untapped potential with their large populations and improving healthcare infrastructure. The integration of advanced technologies such as Artificial Intelligence and machine learning into implantable devices offers avenues for enhanced diagnostic capabilities, personalized therapy, and improved remote monitoring. Miniaturization of devices and the development of less invasive implantation techniques will further improve patient comfort and potentially broaden the eligible patient population. Expansion of clinical indications to include a wider range of OSA severities and exploring novel biomaterials for improved biocompatibility and long-term performance are also key areas of opportunity, promising to drive innovation and market penetration.
- Drivers:
- Increasing prevalence of Obstructive Sleep Apnea (OSA) globally.
- High non-adherence and intolerance rates to Continuous Positive Airway Pressure (CPAP) therapy.
- Technological advancements in implant design and functionality.
- Growing awareness among patients and healthcare professionals about alternative OSA treatments.
- Favorable reimbursement policies in key developed regions.
- Rising disposable incomes and healthcare expenditure in emerging economies.
- Demand for less invasive and more comfortable treatment options.
- Increasing geriatric population prone to OSA.
- Restraints:
- High cost of sleep apnea implants and associated surgical procedures.
- Potential surgical complications and risks.
- Limited long-term clinical data for newer implant technologies.
- Lack of widespread awareness and acceptance in certain regions.
- Stringent regulatory approval processes for novel medical devices.
- Need for specialized training for medical professionals.
- Opportunities:
- Expansion into emerging markets with large patient pools.
- Integration of AI and machine learning for personalized therapy and enhanced monitoring.
- Development of next-generation, miniaturized, and less invasive devices.
- Broadening of clinical indications for implant use.
- Advancements in bioresorbable and biocompatible materials.
- Strategic collaborations and partnerships for market penetration.
- Focus on patient education and advocacy programs.
- Remote patient monitoring and telehealth integration.
- Impact Forces:
- Regulatory landscape and approval timelines.
- Competitive intensity and new market entrants.
- Technological obsolescence and innovation cycles.
- Healthcare spending trends and economic stability.
- Patient preferences and treatment adherence.
- Clinical trial outcomes and evidence generation.
Segmentation Analysis
The Sleep Apnea Implants Market is comprehensively segmented across various parameters to provide a detailed understanding of its dynamics, identifying key areas of growth, demand, and competitive activity. These segmentations allow for a granular analysis of product types, end-user adoption patterns, and geographical influences, enabling stakeholders to pinpoint specific market niches and strategic priorities. The market can be broadly categorized by the type of implant technology, the end-use setting where these procedures are performed, and by the severity of the sleep apnea condition being addressed, each presenting unique characteristics and growth trajectories within the broader market landscape. This multi-dimensional approach ensures a thorough evaluation of market potential and challenges across different dimensions of the sleep apnea treatment paradigm.
- By Product Type:
- Hypoglossal Nerve Stimulation (HNS) Devices
- Palatal Implants (e.g., Pillar Procedure)
- Tongue Base Suspension Systems
- Others (e.g., soft palate stiffening implants, genioglossus advancement devices)
- By End-User:
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Sleep Centers and Clinics
- Specialty ENT Clinics
- By Severity of OSA:
- Moderate Obstructive Sleep Apnea
- Severe Obstructive Sleep Apnea
- By Patient Type:
- CPAP Intolerant Patients
- Patients Seeking Alternative Therapies
- By Region:
- North America
- Europe
- Asia Pacific (APAC)
- Latin America
- Middle East and Africa (MEA)
Value Chain Analysis For Sleep Apnea Implants Market
The value chain for the Sleep Apnea Implants Market begins with the upstream activities involving research and development, raw material procurement, and component manufacturing. This initial phase focuses on developing innovative technologies, sourcing biocompatible materials like titanium, silicone, and advanced polymers, and manufacturing intricate electronic components, batteries, and leads crucial for nerve stimulation devices. Key upstream players include specialized biomaterial suppliers and contract manufacturers providing expertise in micro-electronics and implantable device components. The quality and reliability of these upstream inputs directly impact the safety, efficacy, and longevity of the final implantable product, making robust supplier relationships critical for manufacturers.
Moving downstream, the value chain encompasses the manufacturing, assembly, sterilization, and packaging of the complete implantable systems. Device manufacturers then engage in extensive marketing, sales, and distribution activities to reach healthcare providers. Distribution channels are typically a mix of direct sales forces that target major hospital networks and specialized sleep centers, as well as indirect channels involving third-party distributors and regional partners who have established relationships with smaller clinics and healthcare facilities. These distributors play a vital role in logistical support, inventory management, and often provide local sales and technical support, especially in geographically dispersed markets.
The final stage of the value chain involves the end-users: hospitals, ambulatory surgical centers, and sleep clinics where the implants are surgically placed. Post-surgical follow-up, device activation, and ongoing patient management, often involving sleep specialists and ENT surgeons, complete the patient journey. Direct interaction between manufacturers and key opinion leaders (KOLs) within these end-user segments is crucial for clinical adoption, training, and gathering real-world evidence. The efficacy of both direct and indirect distribution strategies relies heavily on regulatory compliance, effective professional education, and strong clinical support to ensure patient safety and positive outcomes across the entire treatment continuum.
Sleep Apnea Implants Market Potential Customers
The primary potential customers for sleep apnea implants are individuals diagnosed with moderate to severe Obstructive Sleep Apnea (OSA) who are intolerant to or have failed to achieve adequate therapeutic benefits from conventional treatments, most notably Continuous Positive Airway Pressure (CPAP) therapy. This significant subset of the OSA population represents a substantial unmet medical need, as CPAP non-adherence remains a prevalent issue globally, leaving many patients without an effective long-term solution. These patients are often seeking alternative, less cumbersome, and more discreet treatment options that can significantly improve their quality of life, reduce daytime sleepiness, and mitigate the serious health risks associated with untreated sleep apnea, such as cardiovascular disease and metabolic disorders.
Beyond the patients themselves, key buyers and influencers in the adoption of sleep apnea implants include a range of specialized healthcare professionals and institutions. Sleep specialists, pulmonologists, otolaryngologists (ENT surgeons), and neurologists are critical in identifying suitable candidates, making treatment recommendations, and performing the necessary surgical procedures. These medical professionals serve as gatekeepers and educators, guiding patients through the evaluation and treatment process. Their understanding of implant technologies, clinical evidence, and patient selection criteria directly impacts the utilization rates of these devices within their practices.
Furthermore, hospitals, ambulatory surgical centers (ASCs), and dedicated sleep centers constitute essential institutional customers. These facilities provide the infrastructure for diagnosis, surgical implantation, and post-operative care. Hospitals, particularly those with comprehensive sleep disorder programs, are significant purchasers due to their capacity for complex surgical procedures and multidisciplinary patient management. ASCs are increasingly becoming preferred sites for these minimally invasive implant procedures due to their efficiency and cost-effectiveness. The purchasing decisions within these institutions are influenced by factors such as clinical effectiveness, cost-benefit analysis, reimbursement policies, and the overall clinical and economic value that sleep apnea implants offer in addressing a critical public health issue.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 550 Million |
| Market Forecast in 2032 | USD 1150 Million |
| Growth Rate | 10.5% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Inspire Medical Systems Inc., Nyxoah SA, ResMed Inc., Philips Respironics, LivaNova PLC, ImThera Medical Inc. (acquired by LivaNova), Siesta Medical Inc., ReVENT Medical Inc., Zephyr Sleep Technologies, Sommetrics, Inc., Apnicure Inc., GlaxoSmithKline PLC (via acquisition of various respiratory assets), Itamar Medical Ltd. (acquired by ZOLL Medical), MED-EL GmbH, Stryker Corporation, Invibio Biomaterial Solutions, Advanced Bionics (a Sonova company), Cochlear Limited, Biogen Inc., Boston Scientific Corporation. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Sleep Apnea Implants Market Key Technology Landscape
The Sleep Apnea Implants Market is characterized by a dynamic and rapidly evolving technological landscape, primarily centered around innovative approaches to maintain upper airway patency during sleep. The most prominent technology involves neurostimulation, specifically hypoglossal nerve stimulation (HNS), which utilizes a surgically implanted system to deliver mild electrical pulses to the hypoglossal nerve. This stimulation causes the tongue and other upper airway muscles to move forward, preventing airway collapse. Advances in HNS technology focus on miniaturization of components, extended battery life, enhanced sensing capabilities to accurately detect breathing patterns, and algorithms for adaptive, personalized therapy delivery, aiming to maximize efficacy while minimizing patient discomfort and surgical invasiveness.
Another significant technological area encompasses various forms of palatal implants and soft tissue stiffening procedures designed to address specific anatomical contributors to OSA. These technologies involve implanting small, often bioresorbable, structures into the soft palate to increase its rigidity and reduce vibration and collapse. Research in this domain is exploring new biomaterials with improved biocompatibility, degradation profiles, and mechanical properties to ensure long-term stability and effectiveness. The goal is to offer less invasive options for patients with specific palatal-centric airway obstructions, providing an alternative to more extensive traditional surgical interventions like Uvulopalatopharyngoplasty (UPPP).
Beyond core implant technologies, the market is increasingly integrating ancillary digital and diagnostic innovations. Remote monitoring capabilities, often linked with smartphone applications and cloud-based platforms, allow clinicians to track device performance, patient adherence, and physiological parameters from a distance, facilitating proactive patient management and troubleshooting. The burgeoning field of Artificial Intelligence and Machine Learning is also impacting this landscape, with potential applications in refining diagnostic criteria for implant candidacy, optimizing device settings based on individual patient physiology, and enhancing predictive analytics for long-term treatment success. Furthermore, advancements in surgical instrumentation and imaging techniques are making implant procedures safer and more precise, contributing to broader clinical acceptance and patient outcomes.
Regional Highlights
- North America: Dominates the global sleep apnea implants market due to high prevalence of OSA, advanced healthcare infrastructure, significant R&D investments, and favorable reimbursement policies, particularly in the United States. High awareness among both patients and physicians, coupled with a large pool of CPAP-intolerant patients, drives strong adoption.
- Europe: Represents a substantial market with a strong emphasis on clinical evidence and patient safety. Countries like Germany, France, and the UK are leading in adopting these advanced therapies due to robust healthcare systems, an aging population, and increasing awareness of OSA comorbidities. Regulatory harmonization also facilitates market growth.
- Asia Pacific (APAC): Expected to be the fastest-growing region, driven by a large undiagnosed patient population, improving healthcare access, rising disposable incomes, and increasing awareness campaigns regarding sleep disorders. Countries such as China, Japan, India, and Australia are emerging as key markets with growing investments in healthcare infrastructure.
- Latin America: An emerging market for sleep apnea implants, characterized by improving healthcare services and increasing awareness, particularly in countries like Brazil and Mexico. Economic development and rising healthcare expenditure are gradually making advanced treatments more accessible, though market penetration is still relatively low compared to developed regions.
- Middle East and Africa (MEA): This region is experiencing nascent growth, primarily influenced by increasing healthcare spending, growing prevalence of lifestyle diseases contributing to OSA, and rising medical tourism. Adoption is concentrated in affluent countries with advanced medical facilities, but significant opportunities exist as healthcare infrastructure develops across the region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Sleep Apnea Implants Market.- Inspire Medical Systems Inc.
- Nyxoah SA
- ResMed Inc.
- Philips Respironics
- LivaNova PLC
- ImThera Medical Inc.
- Siesta Medical Inc.
- ReVENT Medical Inc.
- Zephyr Sleep Technologies
- Sommetrics, Inc.
- Apnicure Inc.
- ZOLL Medical Corporation (via acquisition of Itamar Medical Ltd.)
- MED-EL GmbH
- Stryker Corporation
- Invibio Biomaterial Solutions
- Advanced Bionics (a Sonova company)
- Cochlear Limited
- Boston Scientific Corporation
- Glaukos Corporation
- Inari Medical, Inc.
Frequently Asked Questions
What are sleep apnea implants and how do they work?
Sleep apnea implants are small, surgically placed medical devices designed to treat Obstructive Sleep Apnea (OSA) by preventing airway collapse during sleep. The most common type, hypoglossal nerve stimulators, work by monitoring breathing patterns and delivering mild electrical pulses to the hypoglossal nerve, which moves the tongue and other upper airway muscles forward, opening the airway. Other types, such as palatal implants, mechanically stiffen the soft palate to reduce obstruction.
Who is a suitable candidate for sleep apnea implant therapy?
Candidates for sleep apnea implants are typically adults diagnosed with moderate to severe Obstructive Sleep Apnea who have not achieved adequate relief or cannot tolerate Continuous Positive Airway Pressure (CPAP) therapy. Specific criteria may include a Body Mass Index (BMI) within a certain range and confirmation of upper airway collapse amenable to implant intervention, often determined through drug-induced sleep endoscopy (DISE).
What are the primary benefits of choosing a sleep apnea implant?
The main benefits of sleep apnea implants include significant improvement in sleep quality, reduction in the Apnea-Hypopnea Index (AHI), and alleviation of OSA symptoms like loud snoring and daytime sleepiness. These devices offer a comfortable and often permanent alternative for CPAP-intolerant patients, leading to enhanced overall quality of life, better cardiovascular health, and reduced risks associated with untreated sleep apnea, without the nightly reliance on external equipment.
Are sleep apnea implants covered by insurance?
Insurance coverage for sleep apnea implants varies by region and specific insurance provider. In many developed countries, including the United States, major private and government insurance plans (such as Medicare) often cover hypoglossal nerve stimulation therapy for eligible patients who meet specific clinical criteria, especially after demonstrating CPAP intolerance. Patients are advised to consult their insurance provider for detailed coverage information and eligibility requirements.
How effective are sleep apnea implants in treating OSA?
Clinical studies demonstrate high effectiveness for sleep apnea implants, particularly hypoglossal nerve stimulation devices, in reducing OSA severity and improving patient outcomes. Many patients experience a significant decrease in their AHI, leading to a substantial reduction in snoring and a marked improvement in daytime function and quality of life. Long-term data continues to show sustained efficacy and high patient satisfaction for appropriately selected candidates.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
