Spinal Muscular Atrophy Treatment Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427357 | Date : Oct, 2025 | Pages : 244 | Region : Global | Publisher : MRU
Spinal Muscular Atrophy Treatment Market Size
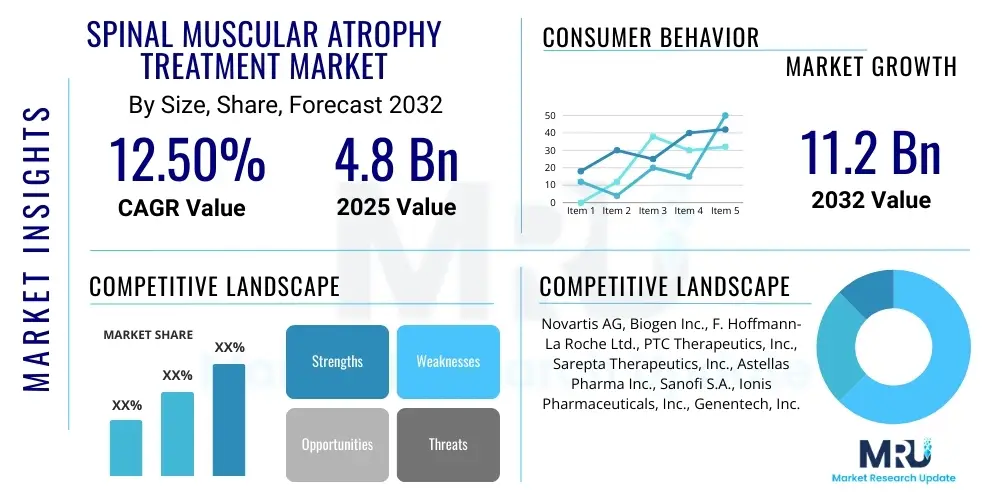
The Spinal Muscular Atrophy Treatment Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2025 and 2032. The market is estimated at USD 4.8 Billion in 2025 and is projected to reach USD 11.2 Billion by the end of the forecast period in 2032.
Spinal Muscular Atrophy Treatment Market introduction
The Spinal Muscular Atrophy (SMA) Treatment Market encompasses a range of innovative therapeutic approaches designed to address the underlying genetic cause and progressive muscle weakness associated with this rare neuromuscular disorder. SMA is characterized by the degeneration of motor neurons in the spinal cord and brainstem, leading to muscle atrophy and weakness. It is primarily caused by a deficiency of the survival motor neuron (SMN) protein, vital for motor neuron function.
Currently available product descriptions include gene therapies, antisense oligonucleotide (ASO) therapies, and small molecule modifiers. These treatments work by either replacing the defective SMN1 gene, increasing the production of the SMN protein from the SMN2 gene, or directly modifying SMN protein levels. Major applications of these treatments span across various types of SMA, including the most severe Type 1 (infantile-onset), Type 2 (intermediate), and Type 3 (juvenile-onset), significantly improving motor function, reducing the need for ventilatory support, and increasing survival rates for affected individuals.
The primary benefits of these advanced therapies include halting or reversing disease progression, enhancing motor milestones, and substantially improving the quality of life for patients. Key driving factors for market growth include the high unmet medical need associated with SMA, robust research and development activities leading to novel therapeutic discoveries, favorable orphan drug designations and accelerated regulatory approvals, increasing global awareness, and improved diagnostic capabilities, particularly through newborn screening programs. These factors collectively contribute to the expansion and accessibility of life-changing treatments for SMA patients worldwide.
Spinal Muscular Atrophy Treatment Market Executive Summary
The Spinal Muscular Atrophy Treatment Market is experiencing rapid evolution driven by significant advancements in gene therapy and oligonucleotide-based treatments. Business trends indicate a strong focus on strategic collaborations, mergers and acquisitions among pharmaceutical and biotechnology companies aimed at expanding product portfolios and geographic reach. Investments in personalized medicine approaches are also prominent, with a shift towards early diagnosis and intervention, particularly through widespread newborn screening programs, which are critically impacting patient identification and treatment initiation rates. This market is characterized by high research and development spending, pushing the boundaries of therapeutic innovation to address remaining challenges in disease management.
Regional trends highlight North America and Europe as dominant markets, primarily due to well-established healthcare infrastructures, high patient awareness, and favorable reimbursement policies for high-cost orphan drugs. However, the Asia Pacific region is rapidly emerging as a significant growth area, fueled by improving healthcare access, increasing healthcare expenditure, and a growing number of diagnostic initiatives. Latin America, the Middle East, and Africa, while currently representing smaller market shares, present substantial future growth opportunities as healthcare systems evolve and access to advanced therapies expands, supported by global humanitarian initiatives and local government investments in rare disease care.
Segmentation trends reveal that gene therapy and antisense oligonucleotide (ASO) therapies currently hold significant market shares due to their established efficacy and approvals for various SMA types. The small molecule segment is also witnessing robust growth, offering advantages in terms of oral administration and broader accessibility. Further, the market is seeing increased focus on therapies targeting different age groups and disease severities, with a burgeoning pipeline of next-generation treatments including gene editing tools and combination therapies. These trends collectively underscore a dynamic market environment dedicated to improving outcomes for SMA patients through diverse and innovative treatment modalities.
AI Impact Analysis on Spinal Muscular Atrophy Treatment Market
Common user questions regarding the impact of Artificial Intelligence (AI) on the Spinal Muscular Atrophy Treatment Market frequently revolve around AIs capabilities in accelerating drug discovery, enhancing diagnostic accuracy, and personalizing treatment strategies. Users are keen to understand how AI can streamline the identification of therapeutic targets, optimize lead compound selection, and predict patient responses to various SMA treatments. There is also significant interest in AIs role in interpreting complex genetic data for improved diagnosis and prognosis, as well as its potential to manage clinical trial data more efficiently. Ethical concerns related to data privacy and equitable access to AI-driven healthcare solutions also emerge, alongside expectations for AI to reduce development costs and improve overall patient outcomes.
- AI accelerates drug discovery by identifying novel therapeutic targets and optimizing lead compounds, reducing the time and cost associated with preclinical research for SMA treatments.
- AI enhances diagnostic accuracy by analyzing genetic sequencing data and neuromuscular imaging, leading to earlier and more precise identification of SMA types and progression.
- AI facilitates personalized medicine by predicting individual patient responses to specific SMA therapies based on genetic profiles and disease biomarkers, tailoring treatment regimens for optimal outcomes.
- AI optimizes clinical trial design and execution through predictive analytics, patient stratification, and real-time monitoring of efficacy and safety data, accelerating the development of new SMA drugs.
- AI-powered patient monitoring systems can track disease progression and treatment effectiveness remotely, providing valuable data for healthcare providers and improving patient management.
DRO & Impact Forces Of Spinal Muscular Atrophy Treatment Market
The Spinal Muscular Atrophy (SMA) Treatment Market is significantly shaped by a confluence of driving forces, formidable restraints, and promising opportunities, all influenced by various impact forces. A primary driver is the profound unmet medical need, given SMAs status as a severe, life-limiting rare disease, which fuels a strong impetus for therapeutic development. The advent of highly effective advanced therapies, including groundbreaking gene therapies and antisense oligonucleotides, has transformed the treatment landscape, offering previously unavailable options for disease modification. Robust investment in research and development, coupled with favorable regulatory pathways such such as orphan drug designations and accelerated approvals, further propels market expansion. Increasing global awareness of SMA, alongside the expansion of newborn screening programs, leads to earlier diagnosis and prompt initiation of these expensive, yet life-saving treatments, significantly impacting market growth.
Despite the revolutionary progress, the market faces several significant restraints. The exceptionally high cost of SMA treatments poses a considerable challenge, often leading to issues with patient access and reimbursement complexities, particularly in regions with less developed healthcare systems or stringent budget constraints. Limited long-term efficacy and safety data for some of the newer therapies, as their clinical histories are still relatively short, contribute to uncertainty for healthcare providers and payers. Potential side effects and complex administration routes, such as intrathecal injections or one-time intravenous infusions, require specialized medical infrastructure and expertise, which are not universally available. Furthermore, the small patient population inherent to rare diseases can sometimes limit the economic feasibility for new entrants and create intense competition among existing players.
Opportunities for growth are abundant within the SMA treatment market. The continuous evolution of therapeutic modalities, including next-generation gene editing technologies like CRISPR, novel small molecules with improved profiles, and combination therapies, promises to address existing limitations and expand treatable populations. Geographical expansion into emerging markets, where diagnosis rates are rising and healthcare infrastructures are improving, represents a significant growth avenue. The expansion of newborn screening programs globally will continue to drive early diagnosis, facilitating prompt treatment initiation and improving overall patient outcomes. Moreover, enhanced patient advocacy and increased funding for rare disease research continue to create a supportive ecosystem for innovation and market penetration. These impact forces, ranging from regulatory support and technological advancements to economic considerations and the influence of patient organizations, collectively dictate the trajectory and accessibility of SMA treatments.
Segmentation Analysis
The Spinal Muscular Atrophy Treatment Market is comprehensively segmented to provide a detailed understanding of its diverse components and drivers. These segmentations are critical for analyzing market dynamics, identifying growth opportunities, and understanding the competitive landscape. Key segments typically include classification by treatment type, which distinguishes between the various therapeutic modalities currently available or in development. Further segmentation often considers the type of SMA being treated, acknowledging the varying clinical presentations and severities of the disease. Additionally, the market is often analyzed by the route of administration, reflecting different patient needs and preferences, and by end-user, illustrating the primary healthcare settings where these treatments are deployed. These analytical approaches allow for a granular view of market trends and patient access.
- By Treatment Type:
- Gene Therapy (e.g., Onasemnogene Abeparvovec)
- Antisense Oligonucleotide (ASO) Therapy (e.g., Nusinersen)
- Small Molecule Therapy (e.g., Risdiplam)
- Pipeline and Emerging Therapies
- By SMA Type:
- SMA Type 1 (Severe Infantile-Onset)
- SMA Type 2 (Intermediate Childhood-Onset)
- SMA Type 3 (Juvenile-Onset)
- Other SMA Types (e.g., Type 0, Type 4)
- By Route of Administration:
- Intravenous
- Intrathecal
- Oral
- By End-User:
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Homecare Settings
- By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Spinal Muscular Atrophy Treatment Market Value Chain Analysis
The value chain for the Spinal Muscular Atrophy (SMA) Treatment Market is complex and multi-faceted, involving numerous stages from initial research and development to patient administration. The upstream segment of the value chain is dominated by intensive research and development activities, which include drug discovery, target identification, preclinical testing, and extensive clinical trials. This phase is highly capital-intensive and requires significant scientific expertise, often involving biotech startups, academic institutions, and large pharmaceutical companies collaborating to bring novel therapies to fruition. Raw material suppliers for active pharmaceutical ingredients (APIs), specialized reagents, and advanced delivery systems, particularly for gene therapy vectors and oligonucleotide synthesis, also form a critical part of the upstream activities, ensuring the foundational components for therapeutic production are available and meet stringent quality standards.
Moving downstream, the value chain encompasses the manufacturing, packaging, and distribution of approved SMA treatments. Manufacturing processes for these advanced therapies are highly specialized and require state-of-the-art facilities compliant with Good Manufacturing Practices (GMP). Distribution channels are critical for ensuring these often temperature-sensitive and high-value products reach healthcare providers efficiently and safely. This involves a network of wholesalers, specialty pharmacies, and logistics providers equipped to handle complex cold chain requirements and direct-to-hospital deliveries for therapies requiring immediate administration. The downstream segment also heavily involves healthcare providers, including hospitals, specialty clinics, and neurologists, who are responsible for prescribing, administering, and monitoring patient outcomes.
The distribution channel within the SMA treatment market typically involves both direct and indirect models. Direct distribution may occur where manufacturers directly supply specialty pharmacies or healthcare institutions, especially for ultra-orphan drugs that require tight control over inventory and cold chain logistics. Indirect channels involve pharmaceutical wholesalers and distributors who act as intermediaries, connecting manufacturers to a broader network of hospitals and specialty clinics. Given the high cost and specialized nature of SMA treatments, reimbursement and patient access programs are integral components of the value chain, often involving insurance providers, government healthcare programs, and patient support organizations, all working to ensure that eligible patients can access these life-changing therapies. This intricate network ensures that the highly specialized treatments for SMA are developed, produced, and delivered effectively to those who need them.
Spinal Muscular Atrophy Treatment Market Potential Customers
The potential customers for Spinal Muscular Atrophy (SMA) treatments primarily consist of the individuals diagnosed with the condition, spanning across all age groups and disease types. This includes infants, children, adolescents, and adults who suffer from the progressive muscle weakness and degeneration characteristic of SMA. For pediatric patients, the decision-making and procurement process often involves their parents or legal guardians, who act as primary advocates and decision-makers in consultation with medical professionals. The urgency for treatment in infantile-onset SMA (Type 1) means that newborns identified through screening programs represent a critical customer segment, emphasizing the importance of rapid diagnosis and therapeutic intervention to preserve motor neuron function.
Beyond the direct patients and their families, key end-users and buyers of SMA treatments include various entities within the healthcare ecosystem. Healthcare providers such as neurologists, pediatric neurologists, geneticists, and general pediatricians are crucial in diagnosing SMA, prescribing appropriate treatments, and managing patient care plans. Hospitals, particularly specialized pediatric hospitals and rare disease centers, serve as major points of administration for therapies like gene therapy and intrathecal injections, making them significant institutional customers. Specialty clinics, rehabilitation centers, and ambulatory surgical centers also play a role in the ongoing management and support of SMA patients.
Additionally, broader stakeholders such as government healthcare programs, public and private insurance providers, and managed care organizations are pivotal customers as they bear a significant portion of the financial burden associated with these high-cost treatments. Their policies on reimbursement, formulary inclusion, and patient access directly impact market penetration and the ability of patients to receive necessary therapies. Patient advocacy groups and non-profit organizations also indirectly influence the market by raising awareness, funding research, and advocating for improved access and affordability, thereby shaping the landscape for potential customers of SMA treatments globally.
Spinal Muscular Atrophy Treatment Market Key Technology Landscape
The Spinal Muscular Atrophy Treatment Market is characterized by a dynamic and rapidly evolving technology landscape, largely driven by advancements in genetic and molecular biology. At the forefront are gene therapy technologies, most notably those utilizing Adeno-Associated Virus (AAV) vectors to deliver functional copies of the SMN1 gene into motor neurons. This technology is designed to provide a one-time, potentially curative treatment, marking a significant breakthrough in rare disease therapy. The continuous refinement of AAV vector design, tropism, and manufacturing processes is a key area of ongoing technological innovation, aiming to improve safety profiles and expand treatment applicability. These advancements are crucial for ensuring efficient gene delivery and expression within target cells.
Another cornerstone of the technological landscape involves Antisense Oligonucleotide (ASO) therapies. These technologies leverage synthetic oligonucleotides to modify RNA splicing of the SMN2 gene, leading to increased production of functional SMN protein. The development of next-generation ASO platforms focuses on enhancing potency, improving cellular uptake, reducing off-target effects, and exploring alternative routes of administration beyond intrathecal delivery. Small molecule modifiers represent a third significant technological pillar, designed to orally cross the blood-brain barrier and increase SMN protein levels by modulating SMN2 gene expression. Research in this area is directed towards discovering and optimizing compounds with higher specificity, better bioavailability, and fewer systemic side effects, offering more convenient dosing regimens for patients.
Beyond direct therapeutic interventions, advanced diagnostic technologies play a critical role. Next-generation sequencing (NGS), array comparative genomic hybridization (aCGH), and quantitative Polymerase Chain Reaction (qPCR) are essential for accurate and early genetic diagnosis of SMA, particularly in newborn screening programs. These technologies ensure that patients are identified promptly, allowing for timely initiation of treatment which is crucial for maximizing therapeutic benefit. Emerging technologies such as CRISPR-based gene editing, while still in early research phases for SMA, hold immense promise for more precise and potentially permanent genetic correction. Furthermore, the integration of Artificial Intelligence and Machine Learning (AI/ML) in drug discovery, biomarker identification, and clinical trial design is increasingly becoming a vital technology for accelerating the development of future SMA treatments by optimizing target validation and predicting therapeutic responses.
Regional Highlights
- North America: This region maintains a dominant position in the Spinal Muscular Atrophy Treatment Market, driven by high research and development expenditures, the presence of major pharmaceutical and biotechnology companies, and favorable reimbursement policies for orphan drugs. Early adoption of advanced therapies and widespread newborn screening programs contribute significantly to market leadership.
- Europe: The European market demonstrates robust growth, supported by strong healthcare infrastructure, increasing awareness among medical professionals, and a growing number of national SMA patient registries. Key countries like Germany, France, and the UK are at the forefront of adopting and reimbursing innovative SMA treatments.
- Asia Pacific: Emerging as a high-growth region, the Asia Pacific market is propelled by improving healthcare access, increasing disposable incomes, and rising awareness of rare diseases. Government initiatives for newborn screening and healthcare infrastructure development in countries like Japan, China, and India are creating significant opportunities for market expansion.
- Latin America: This region is experiencing steady growth, with increasing efforts to improve rare disease diagnosis and access to advanced treatments. While facing challenges in reimbursement and healthcare infrastructure, growing patient advocacy and international collaborations are paving the way for future market development.
- Middle East & Africa: This region is at an nascent stage but holds significant potential for future growth. Increasing healthcare investments, rising awareness of genetic disorders, and the establishment of specialized treatment centers are gradually improving access to SMA therapies, though challenges related to affordability and infrastructure persist.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Spinal Muscular Atrophy Treatment Market.- Novartis AG
- Biogen Inc.
- F. Hoffmann-La Roche Ltd.
- PTC Therapeutics, Inc.
- Sarepta Therapeutics, Inc.
- Astellas Pharma Inc.
- Sanofi S.A.
- Ionis Pharmaceuticals, Inc.
- Genentech, Inc. (a member of Roche Group)
Frequently Asked Questions
What is Spinal Muscular Atrophy and how is it treated?
Spinal Muscular Atrophy (SMA) is a genetic neuromuscular disorder caused by a deficiency in the Survival Motor Neuron (SMN) protein, leading to progressive muscle weakness and degeneration. Treatments available include gene therapy (e.g., Zolgensma), antisense oligonucleotide therapy (e.g., Spinraza), and small molecule therapy (e.g., Evrysdi), which work by either replacing the defective gene or increasing the production of functional SMN protein to modify the disease course.
What are the main types of SMA treatments available?
The primary treatments for SMA encompass three main categories: gene therapy, which delivers a functional copy of the SMN1 gene; antisense oligonucleotide (ASO) therapy, which modulates SMN2 gene splicing to produce more SMN protein; and small molecule therapy, which orally increases SMN protein levels. Each treatment offers a distinct mechanism to address the underlying genetic cause of SMA.
How do the costs of SMA treatments compare?
SMA treatments are among the most expensive therapies globally due to their advanced nature and the rarity of the disease. Gene therapy is typically a one-time treatment often costing several million USD, while ASO and small molecule therapies involve ongoing administration with high annual costs. These high prices reflect significant research and development investments and the profound impact these life-saving interventions have on patient outcomes.
What are the latest advancements in SMA treatment?
Latest advancements in SMA treatment include the expansion of approved therapies for broader age ranges, the development of oral small molecule treatments offering greater convenience, and ongoing research into next-generation approaches such as gene editing techniques (e.g., CRISPR) and combination therapies. There is also a continuous focus on improving diagnostic methods, particularly through expanded newborn screening, and personalizing treatment strategies.
What is the future outlook for the SMA treatment market?
The future outlook for the Spinal Muscular Atrophy treatment market is highly promising, with significant growth expected due to increasing global diagnosis rates driven by newborn screening, a robust pipeline of novel therapies, and greater access to advanced treatments in emerging economies. The market is poised for further expansion through continued innovation in gene therapies, gene editing, and combination approaches, aiming to improve efficacy, broaden patient applicability, and enhance long-term outcomes for SMA patients worldwide.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
