Thrombolytic Drugs Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 428548 | Date : Oct, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Thrombolytic Drugs Market Size
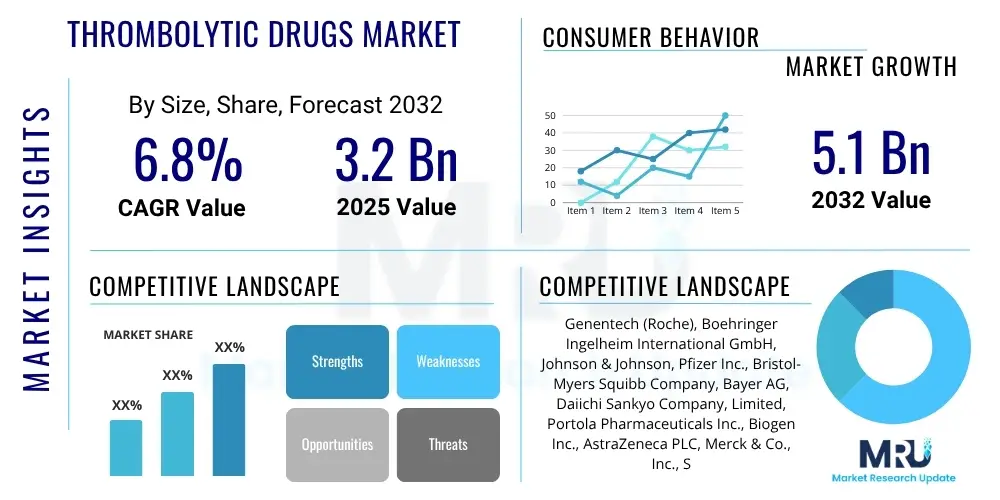
The Thrombolytic Drugs Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.8% between 2025 and 2032. The market is estimated at USD 3.2 billion in 2025 and is projected to reach USD 5.1 billion by the end of the forecast period in 2032.
Thrombolytic Drugs Market introduction
Thrombolytic drugs, often recognized as "clot busters," represent a profoundly critical class of pharmaceutical agents precisely formulated to dissolve hazardous blood clots that impede circulation within blood vessels. Their therapeutic action is initiated by activating plasminogen, an inactive precursor protein found naturally in the body, into its active proteolytic enzyme form, plasmin. Plasmin then systematically degrades fibrin, which constitutes the principal structural component of a thrombus, thereby leading to its dissolution and restoration of blood flow. The primary objective of thrombolytic therapy is to facilitate rapid reperfusion, an urgent necessity in acute cardiovascular and cerebrovascular emergencies such as myocardial infarction (heart attack) and ischemic stroke. In these time-sensitive conditions, every minute lost in treatment initiation can result in irreversible tissue damage and significant functional impairment. The immediate and judicious administration of these drugs is therefore paramount for mitigating long-term disability, preventing necrosis, preserving organ function, and ultimately enhancing patient survival rates, making them indispensable components of modern critical care protocols. Their transformative potential lies in altering the natural history of acute thrombotic events, offering a lifeline to patients facing life-threatening vascular occlusions.
The major applications for thrombolytic drugs span a spectrum of severe thrombotic conditions, including acute myocardial infarction (AMI), ischemic stroke, pulmonary embolism (PE), and deep vein thrombosis (DVT). In cases of AMI, these agents are administered to swiftly reopen blocked coronary arteries, effectively salvaging heart muscle tissue from permanent damage. For ischemic stroke patients, thrombolytics are crucial for rapidly restoring blood supply to the brain, thereby minimizing neurological deficits and improving the chances of a favorable functional recovery. In pulmonary embolism, they address life-threatening clots lodged in the pulmonary arteries, significantly improving respiratory and circulatory function while reducing mortality. Similarly, for deep vein thrombosis, they help dissolve clots in deep veins, primarily in the legs, preventing the development of debilitating post-thrombotic syndrome and reducing the critical risk of a subsequent PE. The profound benefits of thrombolytic therapy encompass a significant reduction in overall patient morbidity and mortality, alongside enhanced functional recovery and an improved quality of life. For instance, in acute ischemic stroke, early intervention can prevent permanent brain damage, underscoring the vital role of these drugs when utilized within stringent therapeutic windows and in accordance with established medical guidelines.
Thrombolytic Drugs Market Executive Summary
The Thrombolytic Drugs Market is currently experiencing robust and dynamic growth, primarily fueled by the escalating global burden of thrombotic disorders and a landscape characterized by continuous pharmaceutical innovation. Current business trends emphatically indicate a strategic imperative for pharmaceutical companies to prioritize the development of next-generation thrombolytics. These advanced agents are engineered to possess enhanced fibrin specificity, a considerably lower propensity for severe bleeding complications, and extended therapeutic windows, thereby addressing critical unmet needs in acute care. To strengthen market presence and broaden therapeutic reach, leading industry players are actively engaged in various strategic maneuvers, including extensive collaborations with both academic research institutions and specialized biopharmaceutical firms, targeted licensing agreements for promising drug candidates, and strategic mergers and acquisitions. These activities foster a highly competitive yet profoundly innovative market environment. Furthermore, an evolving paradigm shift towards personalized medicine is significantly influencing drug discovery and development efforts, with a concerted focus on crafting treatments that are exquisitely tailored to individual patient genetic profiles and clinical characteristics. This personalized approach promises to fundamentally redefine the precision and overall efficacy of thrombolytic interventions, offering more targeted and safer treatment options.
From a regional perspective, North America, particularly the United States, and Europe collectively represent the dominant revenue contributors to the global Thrombolytic Drugs Market. This strong market position is steadfastly supported by highly advanced healthcare infrastructures, a high incidence of cardiovascular and cerebrovascular diseases, robust investment in medical research and development, and highly supportive reimbursement policies that significantly facilitate patient access to these often expensive, life-saving therapies. However, the Asia Pacific region is strategically positioned to demonstrate the most accelerated growth trajectory over the forthcoming forecast period. This rapid expansion is primarily attributable to substantial improvements in healthcare access and infrastructure, a burgeoning and aging population that is increasingly susceptible to thrombotic events, a heightened awareness of acute cardiovascular and cerebrovascular conditions among both the general public and medical professionals, and a significant increase in overall healthcare expenditures across the region. Concurrently, regions such as Latin America, the Middle East, and Africa are also emerging as markets with considerable, though more gradual, growth potential as their respective healthcare systems mature and per capita incomes rise, leading to a greater adoption of advanced medical treatments and technologies. Establishing localized manufacturing capabilities and efficient, cold-chain-compliant distribution networks is becoming an increasingly critical factor for pharmaceutical companies aiming to effectively penetrate and sustain growth within these vibrant emerging economies, addressing local specific needs and market dynamics.
AI Impact Analysis on Thrombolytic Drugs Market
User inquiries concerning the influence of Artificial Intelligence (AI) on the Thrombolytic Drugs Market frequently highlight expectations for significantly improved diagnostic accuracy, enhanced precision in patient selection, and optimized treatment protocols, particularly within time-critical emergency settings. Common themes revolve around how AI can effectively truncate the crucial "door-to-needle" time for acute stroke and myocardial infarction patients, a metric directly correlated with better clinical outcomes and reduced disability. There is substantial interest in AI's capacity to accurately predict individual patient responses to specific thrombolytic agents and to proactively identify individuals at an elevated risk of severe adverse events, such as intracranial hemorrhage, thereby enhancing overall patient safety profiles. Furthermore, a significant portion of the discourse centers on AI's pivotal role in accelerating the discovery and development of novel, safer thrombolytic compounds and its ability to facilitate highly personalized dosing strategies derived from the real-time, comprehensive analysis of vast patient data. While optimism is widespread, concerns often surface regarding critical issues like data privacy and security, the rigorous validation and ethical deployment of complex AI algorithms in clinical practice, and the potential for exacerbating existing healthcare disparities if access to sophisticated AI-driven tools becomes unevenly distributed across different socioeconomic strata or geographic regions, underscoring the need for equitable implementation and robust regulatory oversight.
- AI systems can dramatically improve the speed and precision of diagnosing acute thrombotic conditions by rapidly analyzing medical images (CT, MRI, ECG) with advanced computer vision algorithms, facilitating earlier and more decisive intervention.
- Predictive analytics powered by AI assist clinicians in identifying patients most likely to derive significant therapeutic benefit from thrombolytic therapy and those at elevated risk of complications (e.g., hemorrhagic transformation), enabling more personalized and safer treatment decisions.
- AI algorithms are capable of processing vast amounts of patient data, including medical history, comorbidities, and real-time physiological parameters, to precisely optimize thrombolytic treatment timing, drug selection, and dosage regimens, thereby maximizing efficacy and substantially mitigating adverse outcomes.
- Artificial intelligence accelerates the discovery and development of novel thrombolytic agents through in silico simulation of drug-target interactions, high-throughput virtual screening, and predictive modeling of efficacy and toxicity profiles, significantly shortening the R&D lifecycle.
- Machine learning applications streamline the design and execution of clinical trials for new thrombolytics by identifying optimal patient cohorts, continuously monitoring trial progress, and meticulously analyzing complex datasets to expedite regulatory approval.
- AI contributes to post-market surveillance by continuously analyzing real-world patient data from electronic health records and adverse event reporting systems to detect rare or unforeseen side effects, refining safety profiles and clinical guidelines.
- AI-driven telehealth solutions facilitate rapid preliminary assessment and consultation for individuals exhibiting symptoms of potential thrombotic events, guiding faster patient referral and transport to specialized centers for immediate thrombolytic administration, reducing critical pre-hospital delays.
- Integration of AI with genomic data and biomarker analysis enables highly personalized thrombolytic treatment plans, moving beyond a one-size-fits-all approach for superior individual patient outcomes with minimized risks.
DRO & Impact Forces Of Thrombolytic Drugs Market
The Thrombolytic Drugs Market is dynamically shaped by a critical interplay of powerful driving forces, significant restraining factors, and promising emerging opportunities, which collectively define its trajectory and competitive dynamics. A primary driver for market expansion is the alarming and persistently increasing global burden of cardiovascular diseases (CVDs) and cerebrovascular accidents (strokes), both of which rank as leading causes of mortality and long-term disability worldwide. This epidemiological trend is further exacerbated by a rapidly aging global population, as older individuals are inherently more susceptible to developing thrombotic events due to age-related physiological changes and increased comorbidities. Concurrently, substantial advancements in emergency medical services, coupled with increasingly sophisticated diagnostic capabilities such as enhanced CT perfusion and MRI techniques, enable quicker and more accurate identification of eligible patients, thereby widening the practical application of thrombolytic therapies. Increased public awareness campaigns and targeted medical education initiatives also contribute significantly by promoting early symptom recognition and rapid presentation to healthcare facilities, which enhances the likelihood of timely thrombolytic administration and ultimately improves clinical outcomes, fostering an environment of sustained demand and growth for these critical life-saving medications globally.
Despite these robust driving forces, the market faces notable and inherent restraints that temper its overall growth potential and influence strategic development. The most critical constraint is the inherent risk profile associated with thrombolytic therapy itself, particularly the potential for severe and life-threatening bleeding complications, including intracranial hemorrhage, which necessitates extremely careful patient selection and rigorous, continuous monitoring during and after administration. The narrow therapeutic window, typically spanning only a few hours from symptom onset for maximal efficacy, poses significant logistical and systemic challenges for timely administration in many healthcare settings, especially in regions with limited access to specialized emergency care. Furthermore, the relatively high acquisition cost of advanced thrombolytic agents can present a substantial barrier to widespread access, particularly in resource-constrained healthcare systems and emerging economies, where budget limitations often dictate treatment choices. The stringent and often protracted regulatory approval processes mandated by global health authorities for new pharmaceutical agents also impede the rapid introduction of novel, potentially safer, and more effective thrombolytics to the market. Additionally, the increasing availability and utilization of mechanical thrombectomy, particularly for large vessel occlusion strokes, offer an alternative or complementary treatment modality that, while often synergistic, can also shift treatment paradigms and potentially influence the market share of pharmaceutical thrombolytics in specific patient populations, contributing to overall market complexity and competitive pressures.
Segmentation Analysis
The Thrombolytic Drugs Market is comprehensively segmented across multiple critical dimensions, offering stakeholders a granular and insightful understanding of its intricate dynamics and multifaceted growth prospects. This detailed segmentation is indispensable for discerning overarching market trends, accurately profiling the competitive landscape, and precisely identifying specific unmet clinical needs within distinct therapeutic areas and patient demographics. Such an exhaustive analytical framework empowers pharmaceutical enterprises to strategically refine their research and development portfolios, optimize their targeted marketing campaigns, and efficiently streamline their distribution networks to effectively engage with their intended audiences and maximize market penetration. The primary segmentation methodologies applied in this market analysis encompass drug class, specific application type, preferred route of administration, and the ultimate end-user group. Each category provides unique and invaluable insights into prevailing market behaviors, demand fluctuations, and evolving patient care paradigms, thereby facilitating a more precise and nuanced assessment of the various market drivers, inherent restraints, and burgeoning opportunities that permeate the entire thrombolytic drug value chain. This ultimately enables all relevant stakeholders to make thoroughly informed and strategically sound business decisions, crucial for advancing acute care medicine.
- By Drug Class:
- Urokinase: An enzyme directly converting plasminogen to plasmin.
- Streptokinase: A bacterial protein forming a complex with plasminogen to activate plasmin.
- Alteplase (rt-PA): A recombinant tissue plasminogen activator, highly specific for fibrin-bound plasminogen.
- Reteplase: A modified rt-PA with a longer half-life, allowing bolus administration for AMI.
- Tenecteplase: Another modified rt-PA, offering greater fibrin specificity and single-bolus administration.
- Anistreplase: A prodrug form of streptokinase with slower release.
- Others: Includes investigational or less common agents like Desmoteplase and Saruplase.
- By Application:
- Acute Myocardial Infarction (AMI): For dissolving coronary artery clots during heart attacks.
- Ischemic Stroke: Emergency treatment for brain artery clots.
- Pulmonary Embolism (PE): Therapy for life-threatening clots in pulmonary arteries.
- Deep Vein Thrombosis (DVT): Treatment for clots in deep veins, preventing complications.
- Peripheral Arterial Occlusion: For clearing clots in limb arteries.
- Others: Includes central retinal artery occlusion, acute mesenteric ischemia, and catheter occlusion.
- By Route of Administration:
- Intravenous: Most common, direct injection into a vein for systemic distribution.
- Intra-arterial: Direct injection into an artery near the clot for localized effect.
- Others: May include catheter-directed thrombolysis.
- By End-User:
- Hospitals: Emergency departments, ICUs, Cardiology, Neurology units.
- Specialty Clinics: Cardiovascular, Stroke, Vascular medicine clinics.
- Ambulatory Surgical Centers: For specific adjunctive procedures.
- Cardiac Catheterization Laboratories: For interventional cardiology procedures.
Value Chain Analysis For Thrombolytic Drugs Market
The intricate value chain for the Thrombolytic Drugs Market commences with extensive upstream activities, primarily centered on intensive research and development (R&D). This initial phase involves significant financial and intellectual investment by both pharmaceutical corporations and leading academic institutions to discover novel therapeutic compounds, refine the molecular structures of existing drugs, and conduct rigorous preclinical and early-phase clinical trials to ascertain their fundamental efficacy, safety, and pharmacokinetic properties. A critical component of this upstream segment is the meticulous sourcing and purification of high-quality raw materials, which, given the predominantly biological nature of many thrombolytic agents, often necessitates sophisticated biotechnological processes such as recombinant protein expression and fermentation. Key suppliers in this foundational stage include specialized biotechnology firms providing essential enzymes, recombinant proteins, and other active pharmaceutical ingredients (APIs), as well as contract research organizations (CROs) that offer indispensable support for early-stage drug development, clinical trial design, and execution. The unwavering assurance of the highest standards of quality and purity for these raw materials is absolutely paramount, underscoring the critical, life-saving nature of these medications where any compromise could have severe patient implications and jeopardize therapeutic outcomes.
Progressing downstream, the value chain encompasses the complex and highly regulated process of drug manufacturing. This stage involves sophisticated bioprocessing, precise formulation into stable and administrable forms, stringent quality control measures to ensure batch consistency and purity, and meticulous packaging to maintain product integrity and sterility. Manufacturers must adhere to rigorous Good Manufacturing Practice (GMP) standards set by global regulatory bodies. Following manufacturing, the distribution phase becomes crucial for ensuring timely access to these critical drugs. Distribution often leverages a hybrid model: direct distribution involves dedicated sales forces establishing direct relationships with major hospital networks, emergency medical services, and specialized critical care facilities, ensuring immediate supply and technical support. Indirect distribution, conversely, relies on a vast network of pharmaceutical wholesalers, distributors, and influential group purchasing organizations (GPOs) to reach a broader array of healthcare providers, including smaller clinics, ambulatory surgical centers, and regional pharmacies. The paramount importance of an efficient, secure, and reliable cold chain logistics for many temperature-sensitive thrombolytic agents cannot be overstated, as it directly impacts drug potency and patient safety. Any disruption in these channels can have dire consequences, especially in time-sensitive medical emergencies where rapid availability is non-negotiable. The final segment of the value chain involves the end-users—primarily comprehensive hospitals and specialized clinics—where thrombolytic drugs are administered, with stringent regulatory oversight and post-market surveillance ensuring quality from discovery to patient use.
Thrombolytic Drugs Market Potential Customers
The primary potential customers and ultimate end-users within the Thrombolytic Drugs Market encompass a diverse array of healthcare providers and specialized medical facilities that are equipped to diagnose, manage, and treat acute thrombotic conditions. Foremost among these are large-scale hospitals, particularly their highly specialized emergency departments (EDs), intensive care units (ICUs), and dedicated cardiology and neurology departments. These critical care environments are uniquely structured and staffed to handle the rapid diagnosis and immediate administration of thrombolytic agents, which is an absolute imperative for conditions such as acute myocardial infarction and ischemic stroke, where every minute saved in treatment initiation directly correlates with improved patient outcomes and reduced irreversible damage. Hospitals consistently represent the most substantial procurement segment within the market due to the high volume of critical cases they manage, their comprehensive diagnostic capabilities including advanced imaging, and their capacity for specialized interventional treatments. The unwavering requirement for 24/7 availability of these life-saving medications firmly establishes hospitals as the central pillars of demand within the thrombolytic drugs market, serving as the frontline for acute care interventions across all demographics and geographies. Their operational infrastructure and medical expertise are indispensable for the effective and safe deployment of thrombolytic therapies.
Beyond the extensive network of general and specialized hospital systems, other key potential customers include specialty clinics that maintain a dedicated focus on cardiovascular health, advanced stroke care, and comprehensive vascular medicine. These specialized clinics may administer thrombolytic agents for a spectrum of conditions, ranging from less acute but still serious cases like deep vein thrombosis, to providing critical follow-up care that encompasses managing potential complications or long-term sequelae stemming from thrombotic events. Ambulatory surgical centers (ASCs), while less frequently involved in the initial, immediate administration of thrombolytics for acute emergencies, may nevertheless play a role in specific interventional procedures or in adjunctive capacities where thrombolytics are utilized to facilitate other treatments or prevent complications. Furthermore, governmental healthcare programs, national health services, and various private insurance providers function as influential indirect customers. Their formulary decisions, coverage policies, and intricate reimbursement structures profoundly influence market access, drug uptake rates, and the overall affordability of these critical medications, thereby shaping their availability and accessibility to the broader patient population, significantly impacting market dynamics and patient access to essential care within diverse healthcare systems globally.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 3.2 billion |
| Market Forecast in 2032 | USD 5.1 billion |
| Growth Rate | 6.8% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Genentech (Roche), Boehringer Ingelheim International GmbH, Johnson & Johnson, Pfizer Inc., Bristol-Myers Squibb Company, Bayer AG, Daiichi Sankyo Company, Limited, Portola Pharmaceuticals Inc., Biogen Inc., AstraZeneca PLC, Merck & Co., Inc., Sanofi S.A., Teva Pharmaceutical Industries Ltd., Chiesi Farmaceutici S.p.A., Takeda Pharmaceutical Company Limited, Aspen Pharmacare Holdings Limited, CSL Behring, Lupin Limited, Sun Pharmaceutical Industries Limited, Dr. Reddy's Laboratories Ltd. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Thrombolytic Drugs Market Key Technology Landscape
The technological landscape of the Thrombolytic Drugs Market is characterized by relentless innovation, consistently striving to elevate drug efficacy, enhance patient safety profiles, and optimize delivery mechanisms. Recombinant DNA technology remains a foundational cornerstone, having enabled the industrial-scale production of highly pure, specific, and potent thrombolytic agents. Prominent examples include recombinant tissue plasminogen activators (rt-PAs) such as Alteplase and Tenecteplase, which are crucial for effective clot lysis. This sophisticated biotechnological capability facilitates the consistent and cost-effective manufacturing of these complex protein-based therapeutics. Ongoing advancements in genetic engineering continue to explore intricate modifications to these recombinant proteins, aiming to extend their systemic half-life, minimize potential antigenicity, and further augment their fibrin specificity. The ultimate goal is to strategically reduce systemic bleeding risks while simultaneously maximizing therapeutic benefits. Such meticulous and precise engineering ensures that subsequent generations of thrombolytic agents are not only more targeted in their action but also significantly more potent and safer than their historical predecessors, leading to superior clinical outcomes with fewer complications and broader applicability in acute care medicine.
Beyond the synthesis and engineering of the drugs themselves, substantial technological progress is being observed and actively pursued in novel drug delivery systems. Targeted drug delivery, particularly through the sophisticated application of nanotechnology utilizing nanoparticles or micro-encapsulated systems, represents an area of intensive and highly promising research. The ambition here is to precisely deliver thrombolytic agents directly to the site of the blood clot. This innovative approach aims to achieve maximal local drug concentration where it is needed most, while simultaneously minimizing systemic exposure and consequently reducing the incidence and severity of associated side effects, such as generalized bleeding. Such targeted delivery promises to revolutionize the paradigm of thrombolytic drug administration, offering the compelling potential for lower overall dosages, a narrower therapeutic index for adverse e
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager