Tuberculosis Diagnostics Test Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2025 to 2032 (Financial Impact Analysis)
ID : MRU_ 427928 | Date : Oct, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Tuberculosis Diagnostics Test Market Size
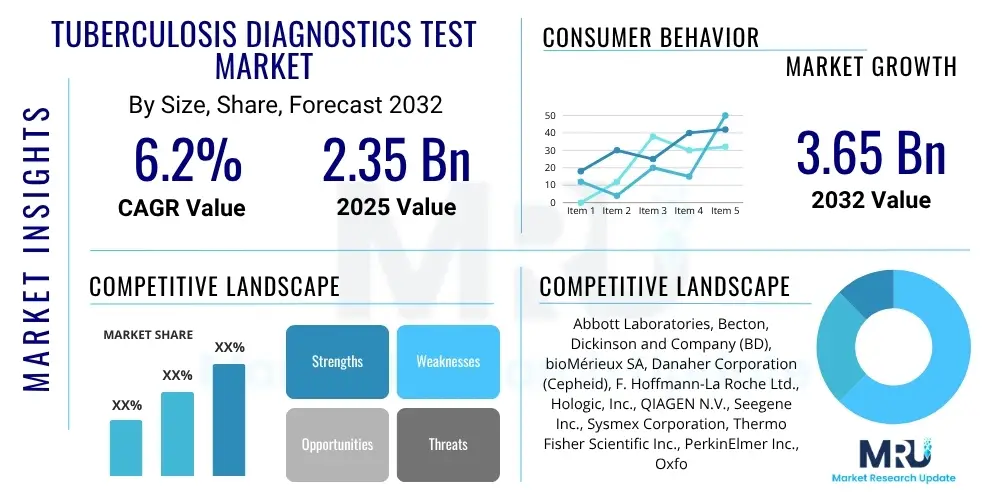
The Tuberculosis Diagnostics Test Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.2% between 2025 and 2032. The market is estimated at USD 2.35 Billion in 2025 and is projected to reach USD 3.65 Billion by the end of the forecast period in 2032.
Tuberculosis Diagnostics Test Market introduction
The Tuberculosis Diagnostics Test Market is a vital component of global public health efforts, focusing on the detection and monitoring of Mycobacterium tuberculosis infection. Tuberculosis, a chronic infectious disease primarily affecting the lungs, remains a leading cause of morbidity and mortality worldwide, necessitating robust diagnostic tools for effective disease management and control. The market encompasses a wide array of diagnostic products, ranging from traditional microscopy and culture methods to advanced molecular and immunodiagnostic assays, each designed to identify the presence of the bacterium, determine drug resistance, or detect latent infection. These tests are applied across various settings, including hospitals, diagnostic laboratories, and point-of-care facilities, to facilitate early diagnosis, prevent disease transmission, and guide appropriate treatment regimens, ultimately contributing to improved patient outcomes and global TB eradication initiatives.
The critical need for rapid and accurate diagnosis of tuberculosis, especially in high-burden regions, drives significant innovation and demand within this market. Products available include sophisticated instruments for automated testing, a diverse range of reagents and consumables essential for test procedures, and supporting software and services that enhance laboratory efficiency and data management. Major applications span the diagnosis of active TB disease, screening for latent TB infection (LTBI) in at-risk populations, and monitoring treatment efficacy to ensure successful patient recovery and prevent relapse. The inherent benefits of these diagnostic tools lie in their ability to enable timely intervention, reduce the spread of infection, and inform public health strategies. The market's growth is predominantly fueled by the persistent high global incidence of TB, the alarming rise of multidrug-resistant (MDR-TB) and extensively drug-resistant (XDR-TB) strains, increasing governmental and non-governmental organization funding for TB control programs, and continuous advancements in diagnostic technologies that offer greater sensitivity, specificity, and speed.
Tuberculosis Diagnostics Test Market Executive Summary
The Tuberculosis Diagnostics Test Market is characterized by dynamic business trends driven by the urgent global health challenge posed by TB. Key business trends include a significant shift towards point-of-care (PoC) testing solutions, increased investment in research and development for novel biomarkers and rapid molecular assays, and growing strategic collaborations among market players, academic institutions, and public health organizations to accelerate product development and market access. The focus is increasingly on developing tests that can accurately detect drug resistance and differentiate between latent and active infections. Regional trends indicate robust growth in the Asia Pacific (APAC) region, primarily due to the high burden of TB in countries like India, China, and Indonesia, coupled with improving healthcare infrastructure and government initiatives. North America and Europe, while having lower incidence rates, lead in technological innovation and adoption of advanced diagnostics, emphasizing highly accurate and automated systems, while Latin America and MEA are emerging markets with increasing awareness and investment in TB control.
Segment trends within the market highlight the dominance and rapid expansion of molecular diagnostics, particularly Nucleic Acid Amplification Tests (NAATs), due to their superior sensitivity, specificity, and ability to detect drug resistance swiftly. This segment is expected to maintain its leading position, driven by continuous technological enhancements and broader acceptance in clinical practice. Immunodiagnostics, such as Interferon-Gamma Release Assays (IGRAs), are gaining traction for latent TB infection screening, offering advantages over traditional tuberculin skin tests. Conversely, traditional methods like sputum smear microscopy and culture-based tests, while still widely used, are seeing incremental improvements aimed at automation and enhanced performance, but their market share is gradually being challenged by faster, more advanced alternatives. The market is also witnessing a trend towards integrated diagnostic platforms that can perform multiple tests simultaneously, improving efficiency and reducing turnaround times across various healthcare settings.
AI Impact Analysis on Tuberculosis Diagnostics Test Market
Users frequently inquire about artificial intelligence's potential to revolutionize the diagnosis and management of tuberculosis, focusing on questions related to enhanced diagnostic accuracy, speed of results, identification of drug resistance patterns, and personalized treatment strategies. Common themes include how AI can improve image analysis for chest X-rays, optimize laboratory workflows, predict disease progression, and assist in epidemiological surveillance. There is significant interest in AI's role in overcoming challenges such as false negatives, lengthy diagnostic processes, and the complexities of drug-resistant TB, with expectations centered on AI-driven solutions offering more efficient, precise, and accessible diagnostics, particularly in resource-limited settings.
- AI-powered image analysis for rapid and accurate interpretation of chest X-rays, reducing reliance on expert radiologists.
- Machine learning algorithms for early detection of TB from complex clinical data, including patient symptoms, demographic information, and biomarkers.
- AI-driven analysis of genomic sequencing data to quickly identify drug-resistant TB strains and predict effective treatment regimens.
- Automation of laboratory processes and workflow optimization using AI, leading to faster turnaround times and reduced human error.
- Development of novel diagnostic tools through AI-accelerated discovery of new biomarkers for both active and latent TB.
- Enhancement of epidemiological surveillance by AI models predicting outbreak patterns and identifying high-risk populations for targeted interventions.
- Personalized treatment recommendations for TB patients by analyzing individual patient data, drug efficacy, and resistance profiles.
- Support for point-of-care diagnostics through AI-enabled portable devices for rapid, on-site testing and interpretation in remote areas.
DRO & Impact Forces Of Tuberculosis Diagnostics Test Market
The Tuberculosis Diagnostics Test Market is significantly shaped by a confluence of driving factors, restrictive challenges, and emerging opportunities, all interacting with broader impact forces. Key drivers include the persistently high global incidence and prevalence of tuberculosis, particularly in developing nations, creating an ongoing demand for diagnostic solutions. The alarming rise of multidrug-resistant (MDR-TB) and extensively drug-resistant (XDR-TB) strains globally necessitates rapid and accurate drug susceptibility testing, further fueling market growth. Moreover, increasing investments in TB control programs by governments and international organizations, coupled with a growing focus on early diagnosis and treatment to curb transmission, act as powerful market accelerators. Technological advancements, leading to more sensitive, specific, and rapid diagnostic platforms, also significantly drive market expansion by improving accessibility and efficiency of testing.
However, the market faces several significant restraints. The high cost associated with advanced molecular diagnostic tests and sophisticated laboratory infrastructure can be prohibitive for many low- and middle-income countries, where the TB burden is highest. Limited access to advanced diagnostic facilities in remote and underserved areas, coupled with a lack of skilled personnel to operate complex equipment, hinders market penetration. Furthermore, issues such as the suboptimal sensitivity and specificity of some existing tests, particularly for extrapulmonary TB or in immunocompromised patients, contribute to diagnostic delays and challenges. Regulatory hurdles and the time-consuming process of test validation and approval also pose barriers to rapid market entry and widespread adoption of new technologies.
Despite these restraints, substantial opportunities exist for market players. The development of innovative point-of-care (PoC) diagnostic solutions offers a significant avenue for growth, enabling rapid testing outside traditional laboratory settings and improving access in resource-limited areas. The integration of artificial intelligence (AI) and machine learning (ML) for enhanced image analysis, data interpretation, and biomarker discovery presents a transformative opportunity to improve diagnostic accuracy and speed. Furthermore, the exploration of novel biomarkers for early and non-invasive detection of both active and latent TB, along with the expansion into emerging markets with high unmet needs, represent key growth frontiers. Impact forces such as evolving public health policies emphasizing universal access to diagnosis, stringent regulatory frameworks pushing for higher test performance, and increasing public awareness campaigns about TB contribute to shaping the market landscape, compelling continuous innovation and adaptation.
Segmentation Analysis
The Tuberculosis Diagnostics Test Market is extensively segmented to reflect the diverse range of technologies, products, applications, and end-users contributing to its overall landscape. This segmentation provides a granular view of market dynamics, revealing specific growth drivers and competitive intensities within each category. Understanding these segments is crucial for stakeholders to identify key areas for investment, product development, and market penetration strategies. The market can be broadly categorized by the type of diagnostic test employed, the nature of the product offered, the specific clinical application, and the primary end-users who utilize these diagnostic solutions, each representing distinct operational characteristics and market demands.
A detailed analysis of these segments illustrates the market's current structure and future trajectory. For instance, the segmentation by test type highlights the shift from traditional, often slower methods, towards rapid, high-throughput molecular diagnostics that provide faster and more accurate results, particularly for drug resistance. Similarly, the product segmentation differentiates between instruments, which represent significant capital investments, and reagents and consumables, which drive recurring revenue. These categorizations are vital for market participants to tailor their offerings and target specific niches, ensuring that diagnostic solutions meet the varied needs of a global population grappling with tuberculosis, ranging from basic screening in rural clinics to advanced drug susceptibility testing in specialized referral laboratories.
By Test Type
The segmentation by test type is fundamental, reflecting the varied scientific approaches used to detect Mycobacterium tuberculosis. This category includes traditional methods such as sputum smear microscopy and culture-based tests, which have been cornerstones of TB diagnosis for decades due to their cost-effectiveness and accessibility, particularly in low-resource settings. While these methods are widely utilized, they often suffer from limitations in sensitivity, specificity, and lengthy turnaround times, necessitating continuous innovation.
In contrast, advanced diagnostic test types, primarily Nucleic Acid Amplification Tests (NAATs), have gained significant prominence. NAATs offer superior sensitivity and specificity, providing rapid results and the ability to detect drug-resistant strains directly from clinical samples. Immunodiagnostics, including Tuberculin Skin Tests (TST) and Interferon-Gamma Release Assays (IGRAs), play a crucial role in screening for latent TB infection, with IGRAs offering improved specificity over TSTs. The market also includes radiography, primarily chest X-rays, often used as a preliminary screening tool or to assess disease severity, and other emerging diagnostic approaches leveraging novel biomarkers.
- Nucleic Acid Amplification Tests (NAATs)
- PCR-based Tests
- LAMP-based Tests
- CRISPR-based Diagnostics
- Sputum Smear Microscopy
- Ziehl-Neelsen (ZN) Staining
- Fluorescence Microscopy
- Culture-Based Tests
- Liquid Culture Systems (e.g., MGIT)
- Solid Culture Media (e.g., Lowenstein-Jensen)
- Immunodiagnostics
- Tuberculin Skin Test (TST)
- Interferon-Gamma Release Assays (IGRAs)
- Radiography
- Chest X-ray
- CT Scans
- Other Diagnostic Tests
- Line Probe Assays (LPAs)
- Rapid Diagnostic Tests (RDTs)
- Serological Tests (limited use)
By Product
The market's product segmentation highlights the distinct components required for tuberculosis diagnostics, spanning instruments, reagents & consumables, and supporting software & services. Instruments form the foundational infrastructure, encompassing a wide range of devices from basic microscopes and incubators to sophisticated automated molecular diagnostic platforms and robotic systems. These instruments represent significant capital investment for healthcare facilities and laboratories, with technological advancements focusing on automation, higher throughput, and multiplexing capabilities to enhance efficiency and accuracy.
Reagents and consumables constitute the recurring expenditure in TB diagnostics, essential for every test performed. This segment includes DNA/RNA extraction kits, PCR reagents, culture media, staining solutions, antibodies, and various disposables such as test tubes, plates, and swabs. The quality and availability of these consumables directly impact test reliability and workflow. As molecular diagnostics become more prevalent, the demand for high-quality, specific reagents designed for these advanced platforms continues to grow, driving innovation in reagent formulation and stability. Software and services encompass the digital and support infrastructure, including laboratory information management systems (LIMS), data analysis software for genomic sequencing, telemedicine platforms for remote interpretation, and comprehensive training and maintenance services for diagnostic equipment.
- Instruments
- Automated Molecular Diagnostic Systems
- Automated Microscopy Systems
- Culture Systems
- Radiography Devices
- Reagents & Consumables
- Molecular Reagents (e.g., NAAT kits, extraction kits)
- Culture Media & Supplements
- Stains & Dyes
- IGRAs Kits
- TST Antigens
- Other Consumables (e.g., collection tubes, slides)
- Software & Services
- Laboratory Information Management Systems (LIMS)
- Data Analysis Software
- Maintenance & Support Services
- Training & Education
By Application
The application segmentation delineates the specific clinical contexts in which tuberculosis diagnostics tests are utilized, primarily distinguishing between the detection of active tuberculosis (TB) and the screening for latent tuberculosis infection (LTBI). Diagnosing active TB is critical for immediate patient management, preventing disease progression, and curbing community transmission. Tests for active TB focus on identifying the presence of Mycobacterium tuberculosis in clinical samples, often with an emphasis on rapid detection and drug susceptibility testing to guide appropriate treatment initiation. This segment drives the demand for sensitive and specific tests that can differentiate TB from other respiratory illnesses and quickly identify drug-resistant strains.
Conversely, screening for latent TB infection is crucial for preventing the development of active disease in individuals who are infected but not yet symptomatic. This application is particularly important in high-risk populations, such as close contacts of active TB patients, immunocompromised individuals, and healthcare workers. Tests for LTBI, primarily IGRAs and TSTs, aim to identify the immunological response to TB bacteria. Effective LTBI screening programs are vital for public health strategies aimed at reducing the overall burden of tuberculosis by proactively treating individuals before they develop active disease, thereby breaking the chain of transmission. Both applications represent distinct but interconnected facets of global TB control efforts, each requiring tailored diagnostic approaches and tools.
- Active Tuberculosis (TB) Diagnosis
- Pulmonary TB Diagnosis
- Extrapulmonary TB Diagnosis
- Drug Resistance Testing
- Latent Tuberculosis Infection (LTBI) Screening
- High-Risk Population Screening
- Contact Tracing
- Pre-treatment Screening (e.g., before immunosuppressive therapy)
By End-User
The end-user segmentation highlights the primary institutions and healthcare providers that utilize tuberculosis diagnostic tests. Hospitals represent a significant end-user segment, given their role in inpatient care, emergency services, and comprehensive diagnostic facilities. In hospitals, TB diagnostic tests are crucial for both admitted patients with suspected TB and for screening healthcare workers. The demand from hospitals often leans towards rapid, integrated platforms that can provide quick turnaround times to facilitate timely isolation, treatment, and infection control measures.
Diagnostic laboratories, including private, public, and reference laboratories, form another major end-user category. These facilities specialize in processing a high volume of samples and often possess advanced molecular diagnostic capabilities. Public health laboratories play a critical role in surveillance, outbreak investigation, and confirming drug-resistant TB cases. Research institutes, while not directly involved in routine patient diagnosis, are vital for test development, validation, and understanding TB pathogenesis, driving the demand for innovative diagnostic platforms and reagents. The varied needs across these end-user segments influence product development, distribution channels, and service offerings within the TB diagnostics market.
- Hospitals
- General Hospitals
- Specialty Hospitals (e.g., infectious disease hospitals)
- Diagnostic Laboratories
- Private Diagnostic Labs
- Public Diagnostic Labs
- Reference Laboratories
- Public Health Laboratories
- Research Institutes & Academia
- Academic Research Centers
- Pharmaceutical & Biotech R&D
- Other End-Users (e.g., Physician Offices, Clinics, NGOs)
Value Chain Analysis For Tuberculosis Diagnostics Test Market
The value chain for the Tuberculosis Diagnostics Test Market is complex, involving multiple stages from initial research and development to final patient diagnosis, illustrating the journey of diagnostic products and services. The upstream analysis begins with extensive research and development (R&D) efforts, where biotechnology and pharmaceutical companies, often in collaboration with academic institutions, focus on discovering novel biomarkers, developing new assay technologies, and improving existing diagnostic platforms. This stage also involves sourcing raw materials and key components, such as enzymes, antibodies, nucleic acid probes, and specialized chemicals, from various suppliers. Manufacturing then transforms these raw materials into diagnostic kits, instruments, and reagents, adhering to strict quality control and regulatory standards.
The midstream involves the distribution and logistics network. Once manufactured, diagnostic products are transported to various markets globally, requiring robust supply chain management, warehousing, and inventory control. Distributors, often specialized in medical devices and diagnostics, play a crucial role in reaching diverse end-users, including hospitals, diagnostic laboratories, and public health programs. They manage sales, marketing, and technical support, ensuring that products are available and properly utilized. Regulatory bodies also play a significant role at this stage, with approvals and certifications being essential for market access and product compliance across different regions.
Downstream analysis focuses on the end-users and the ultimate delivery of diagnostic services. Hospitals and diagnostic laboratories utilize these tests for patient diagnosis, treatment monitoring, and screening, integrating them into their clinical workflows. Public health laboratories are critical for surveillance and confirming complex cases, especially drug-resistant TB. The distribution channels can be direct, where manufacturers sell directly to large institutional clients or through their own sales forces, or indirect, leveraging a network of third-party distributors, wholesalers, and even online platforms for broader market reach. Direct channels offer greater control over sales and customer relationships, while indirect channels provide wider geographical coverage and access to smaller clients. The effectiveness of the entire value chain is paramount for ensuring timely and accurate TB diagnosis, which is fundamental to global TB control efforts.
Tuberculosis Diagnostics Test Market Potential Customers
The potential customers for the Tuberculosis Diagnostics Test Market are diverse and span various segments of the healthcare ecosystem, reflecting the broad impact of tuberculosis on public health. The primary end-users and buyers of these products and services include hospitals, which rely on diagnostic tests for patient admissions, inpatient management, and infection control. These institutions often require a range of tests, from rapid screening tools in emergency departments to advanced molecular diagnostics in their microbiology laboratories, catering to both active TB diagnosis and latent TB screening for at-risk patient populations and healthcare workers. Their demand is driven by the need for quick, accurate, and often automated solutions to manage patient flow and ensure timely treatment.
Diagnostic laboratories, encompassing private, public, and reference laboratories, constitute another major customer segment. These facilities are often specialized in processing a high volume of samples and have the infrastructure for sophisticated molecular and culture-based testing. Public health laboratories, in particular, serve as crucial buyers, as they are instrumental in national TB surveillance programs, confirming drug-resistant cases, and implementing broader screening initiatives. These laboratories often prioritize cost-effectiveness, high throughput, and the ability to integrate with existing public health reporting systems. Additionally, research institutes and academic centers are key customers, albeit for different reasons, as they purchase diagnostic components and platforms for ongoing research, test development, and validation studies aimed at advancing the field of TB diagnostics. These customers seek cutting-edge technologies and specialized reagents for their scientific endeavors.
Furthermore, private clinics and physician offices increasingly represent a growing segment, especially with the rise of point-of-care (PoC) diagnostic solutions. These smaller healthcare settings require user-friendly, rapid, and often cartridge-based tests that can be performed without extensive laboratory infrastructure or specialized training. Non-governmental organizations (NGOs) and international health agencies, such as the World Health Organization (WHO) and Médecins Sans Frontières, are significant purchasers, especially for deployment in high-burden, resource-limited countries where they manage TB control and treatment programs. Their procurement decisions are heavily influenced by factors such as affordability, ease of use, robustness, and regulatory approval by global health authorities. Pharmaceutical companies also utilize TB diagnostics for clinical trials, particularly in drug development programs targeting new TB drugs, requiring reliable and standardized tests for patient enrollment and efficacy monitoring.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2025 | USD 2.35 Billion |
| Market Forecast in 2032 | USD 3.65 Billion |
| Growth Rate | 6.2% CAGR |
| Historical Year | 2019 to 2023 |
| Base Year | 2024 |
| Forecast Year | 2025 - 2032 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Abbott Laboratories, Becton, Dickinson and Company (BD), bioMérieux SA, Danaher Corporation (Cepheid), F. Hoffmann-La Roche Ltd., Hologic, Inc., QIAGEN N.V., Seegene Inc., Sysmex Corporation, Thermo Fisher Scientific Inc., PerkinElmer Inc., Oxford Immunotec, Hain Lifescience GmbH (part of Bruker), Molbio Diagnostics Pvt. Ltd., TB Alliance, Sanofi, Siemens Healthineers, FUJIFILM Wako Pure Chemical Corporation, MycoDot |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Tuberculosis Diagnostics Test Market Key Technology Landscape
The Tuberculosis Diagnostics Test Market is continually shaped by advancements in various scientific and engineering disciplines, leading to a dynamic technological landscape. Molecular diagnostics stand at the forefront, leveraging sophisticated techniques such as Polymerase Chain Reaction (PCR), Loop-mediated Isothermal Amplification (LAMP), and Nucleic Acid Amplification Tests (NAATs) to rapidly detect Mycobacterium tuberculosis DNA or RNA. These technologies offer high sensitivity and specificity, enabling quick identification of both active infection and drug resistance markers, which is crucial for timely treatment initiation and containment of drug-resistant strains. The development of multiplex PCR assays and next-generation sequencing (NGS) is further enhancing the ability to simultaneously detect multiple pathogens and characterize resistance profiles with unprecedented detail.
Beyond molecular methods, advancements in immunodiagnostics, particularly Interferon-Gamma Release Assays (IGRAs), continue to refine the detection of latent tuberculosis infection. Newer versions of IGRAs aim for improved performance and simpler workflows. Automated microscopy and digital pathology are transforming traditional sputum smear analysis by integrating image processing algorithms and artificial intelligence (AI) to enhance detection accuracy and reduce manual labor, especially in high-volume settings. The emergence of CRISPR-based diagnostics represents a cutting-edge frontier, offering the potential for highly specific, rapid, and cost-effective point-of-care diagnostic tools with minimal infrastructure requirements.
Furthermore, the integration of microfluidics and lab-on-a-chip technologies is paving the way for highly portable and integrated diagnostic devices suitable for point-of-care (PoC) testing in resource-limited environments. These systems are designed to minimize sample preparation and reagent handling, delivering rapid results directly where patients are. The ongoing research into novel biomarkers, including host response markers and bacterial antigens in various biological fluids, is also a critical area of technological innovation. These efforts aim to develop non-sputum-based tests that can overcome the challenges associated with traditional sample collection and improve diagnosis in difficult-to-diagnose populations, such as children and individuals with extrapulmonary TB. The convergence of these technological innovations is driving the market towards more accessible, accurate, and efficient diagnostic solutions for tuberculosis globally.
Regional Highlights
- North America: This region, comprising the United States and Canada, represents a mature market with a strong emphasis on advanced diagnostic technologies and sophisticated healthcare infrastructure. While TB incidence rates are relatively low, there is a high adoption of premium, rapid molecular diagnostics and immunodiagnostic tests, driven by robust research and development activities, significant healthcare expenditure, and a focus on latent TB screening in at-risk populations. The presence of key market players and a strong regulatory framework foster continuous innovation and market growth. The region often leads in the development and early adoption of cutting-edge diagnostic solutions, including AI-powered platforms and CRISPR-based assays.
- Europe: The European market, including countries like Germany, the UK, France, and Italy, demonstrates a balanced approach between traditional and advanced diagnostic methods. Stringent public health policies aimed at TB eradication, coupled with a well-developed healthcare system, drive the demand for accurate and reliable tests. The region focuses on effective contact tracing and latent TB screening. The presence of several prominent diagnostic companies and collaborations with global health initiatives contribute to market growth. However, varying healthcare reimbursement policies and regulatory landscapes across countries can influence market dynamics and product uptake, with a notable push for integrated diagnostic solutions.
- Asia Pacific (APAC): The APAC region, encompassing high-burden countries such as India, China, Indonesia, and the Philippines, is the largest and fastest-growing market for TB diagnostics. This growth is primarily attributed to the high prevalence of tuberculosis, increasing public health awareness, rising healthcare expenditure, and improving diagnostic infrastructure. Government initiatives and international funding to combat TB, particularly drug-resistant forms, are major drivers. The demand is strong for both cost-effective traditional tests and advanced molecular diagnostics, with a growing focus on point-of-care solutions to address vast geographical and demographic disparities in healthcare access.
- Latin America: Countries like Brazil, Mexico, and Argentina constitute the Latin American market, which is characterized by a moderate TB burden and increasing efforts to improve diagnostic capabilities. Market growth is driven by government investments in public health programs, rising awareness, and the gradual adoption of modern diagnostic techniques. Challenges include economic disparities and infrastructure limitations in certain areas, which create demand for affordable and robust diagnostic solutions. Strategic partnerships and international aid play a significant role in expanding access to TB diagnostics in this region.
- Middle East and Africa (MEA): The MEA region faces a significant challenge from tuberculosis, particularly in sub-Saharan Africa, which has some of the highest incidence rates globally. This makes it a region with immense unmet needs and significant growth potential. Market drivers include increasing investments from international organizations and philanthropic foundations, growing awareness campaigns, and improvements in healthcare infrastructure. The demand is particularly high for rapid, cost-effective, and easy-to-use point-of-care diagnostics suitable for remote and resource-limited settings. Addressing the high burden of HIV/TB co-infection also shapes the diagnostic requirements in this region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Tuberculosis Diagnostics Test Market.- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- bioMérieux SA
- Danaher Corporation (Cepheid)
- F. Hoffmann-La Roche Ltd.
- Hologic, Inc.
- QIAGEN N.V.
- Seegene Inc.
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- PerkinElmer Inc.
- Oxford Immunotec
- Hain Lifescience GmbH (part of Bruker)
- Molbio Diagnostics Pvt. Ltd.
- TB Alliance
- Sanofi
- Siemens Healthineers
- FUJIFILM Wako Pure Chemical Corporation
- MycoDot
Frequently Asked Questions
Analyze common user questions about the Tuberculosis Diagnostics Test market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the current growth rate of the Tuberculosis Diagnostics Test Market?
The Tuberculosis Diagnostics Test Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.2% between 2025 and 2032, driven by increasing global TB prevalence and diagnostic advancements.
Which types of diagnostic tests are most prevalent in the market?
Molecular diagnostics, particularly Nucleic Acid Amplification Tests (NAATs), are increasingly prevalent due to their high sensitivity, specificity, and rapid detection of drug resistance. Traditional smear microscopy and culture-based tests also remain widely used.
How does AI impact the Tuberculosis Diagnostics Test Market?
AI is significantly impacting the market by enhancing diagnostic accuracy in chest X-ray interpretation, accelerating drug resistance identification through genomic analysis, and optimizing laboratory workflows for faster, more efficient TB testing.
What are the primary drivers for market growth?
Key drivers include the high global incidence of TB, the rise of drug-resistant strains, increased government funding for TB control programs, and continuous technological advancements leading to more effective diagnostic solutions.
Which region holds the largest market share for TB diagnostics?
The Asia Pacific (APAC) region currently holds the largest market share and is experiencing the fastest growth, primarily due to the high burden of TB in countries like India and China, coupled with improving healthcare infrastructure.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
