Ortho Pediatric Devices Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 437943 | Date : Dec, 2025 | Pages : 245 | Region : Global | Publisher : MRU
Ortho Pediatric Devices Market Size
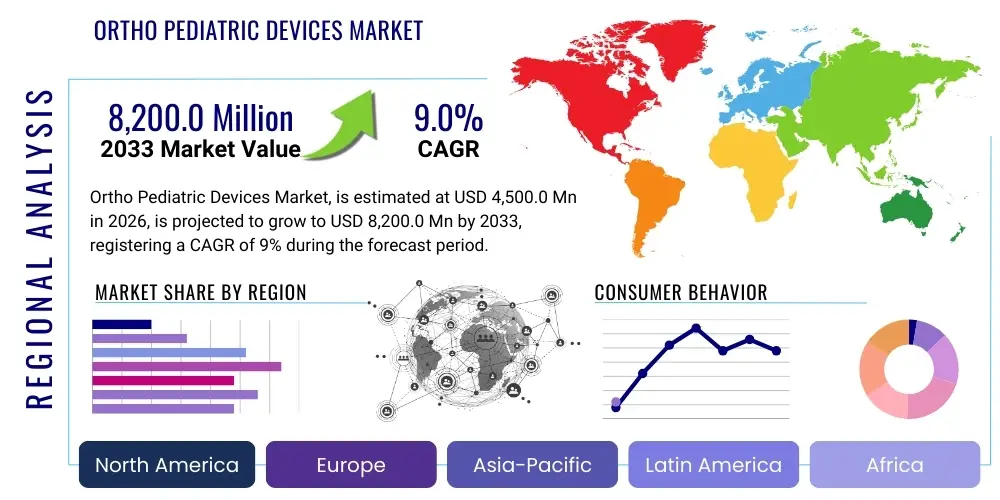
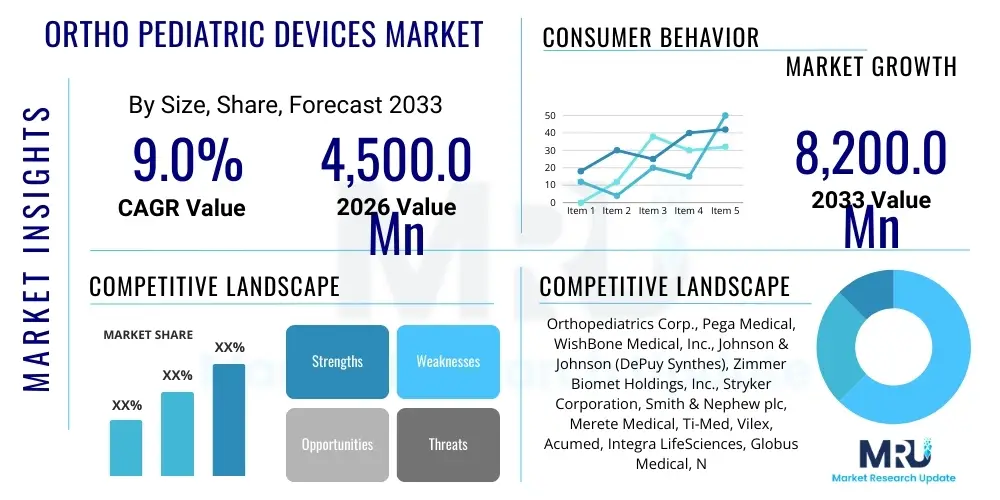
The Ortho Pediatric Devices Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.0% between 2026 and 2033. The market is estimated at USD 4,500.0 million in 2026 and is projected to reach USD 8,200.0 million by the end of the forecast period in 2033.
Ortho Pediatric Devices Market introduction
The Ortho Pediatric Devices Market encompasses specialized medical devices designed for the diagnosis, treatment, and management of musculoskeletal conditions unique to the pediatric population, ranging from neonates to adolescents. These devices address developmental deformities, traumatic injuries, congenital anomalies, and conditions related to growth plates, requiring distinct biomechanical and material properties compared to adult orthopedic devices. Key product categories include internal fixation devices, external fixators, spine systems specifically tailored for scoliosis correction in growing patients, and sophisticated bracing technologies. The necessity for these tailored solutions arises from the continuous growth and remodeling characteristics of the pediatric skeleton, which influence treatment efficacy and long-term patient outcomes, demanding miniaturization, bioresorbable materials, and minimally invasive design principles to reduce surgical morbidity and recovery time.
Major applications for orthopedic pediatric devices span complex areas such as trauma and fracture management, scoliosis and kyphosis correction, limb length discrepancy treatment, and the management of chronic conditions like cerebral palsy and juvenile idiopathic arthritis requiring surgical intervention. The primary benefit of specialized pediatric orthopedics is the ability to provide durable, effective, and growth-friendly treatments that minimize long-term complications such as growth arrest or fixation failure. Furthermore, these devices often incorporate features that allow for adjustability or incremental correction, critical for accommodating the rapid changes in skeletal dimensions characteristic of childhood and adolescence, ensuring optimal function as the child matures.
Driving factors propelling this market include the global increase in birth rates coupled with a greater awareness among clinicians regarding the superior outcomes offered by pediatric-specific implants over off-label adult devices. Technological advancements, particularly in bioresorbable fixation materials and guided growth techniques (such as the use of growth-friendly spinal systems), are continually expanding the scope of treatable conditions and improving procedural safety. Additionally, the rising incidence of childhood obesity leading to increased rates of orthopedic trauma and slipped capital femoral epiphysis (SCFE), combined with improved diagnostics capabilities in congenital disorders, necessitate a robust supply of specialized orthopedic tools and implants, thereby driving sustained market expansion across developed and developing economies.
Ortho Pediatric Devices Market Executive Summary
The Ortho Pediatric Devices Market exhibits robust growth driven by advancements in material science and surgical techniques prioritizing minimally invasive and growth-sparing approaches. Business trends highlight strategic investments in specialized product lines, increased merger and acquisition activity focused on consolidating niche expertise, and significant expenditure on research and development aimed at creating personalized and 3D-printed orthopedic solutions for highly complex pediatric cases. Leading companies are focusing on securing regulatory approvals for next-generation implants designed specifically for small anatomies and fragile growth plates, recognizing the lucrative, yet clinically demanding, nature of this specialty. Furthermore, global supply chain optimization is becoming critical to ensure the availability of highly specialized, low-volume instruments and implants across geographically diverse healthcare settings.
Regional trends indicate North America and Europe currently dominate the market, primarily due to established healthcare infrastructure, high healthcare expenditure, favorable reimbursement policies for pediatric procedures, and the early adoption of advanced surgical robotics and navigation systems in children's hospitals. However, the Asia Pacific region is anticipated to demonstrate the highest growth rate during the forecast period. This accelerated growth is attributed to massive population bases, rising awareness regarding musculoskeletal deformities, improving economic conditions facilitating better access to complex medical treatments, and concerted government efforts in countries like India and China to enhance pediatric healthcare services. The expansion of specialty pediatric hospitals in developing nations is significantly increasing the patient pool requiring sophisticated orthopedic intervention.
Segmentation trends reveal that the Internal Fixation Devices segment, particularly those used for trauma and fracture management, holds a commanding market share due to the high frequency of these injuries in active children. The Spinal Deformity Correction segment is poised for rapid expansion, driven by innovations in magnetically controlled growing rods (MCGRs) and tethering systems (VBT) that offer alternatives to traditional, invasive fusion surgeries. End-user analysis underscores hospitals and large academic medical centers as the primary consumers, although the trend towards specialized outpatient orthopedic clinics for non-complex procedures is gaining traction. The overarching segment trend emphasizes safety, minimizing revision surgeries, and utilizing materials that degrade harmlessly or adapt seamlessly with the child's growing body, such as bioresorbable screws and plates, which mitigate the need for secondary removal procedures.
AI Impact Analysis on Ortho Pediatric Devices Market
Common user inquiries concerning the impact of Artificial Intelligence (AI) in the Ortho Pediatric Devices Market primarily revolve around the optimization of surgical planning, the potential for personalized implant design, and the ethical considerations regarding data use in vulnerable patient populations. Users frequently ask about AI's role in improving the accuracy of deformity correction measurements, predicting pediatric growth trajectories to guide intervention timing, and enhancing the precision of robotic-assisted procedures in small operative fields. Key expectations center on AI models that can analyze large datasets of pediatric skeletal growth patterns to inform better implant sizing and placement, thereby minimizing errors and improving long-term functional outcomes. Concerns often address regulatory hurdles, the validation of AI algorithms for rare pediatric conditions, and ensuring equitable access to these sophisticated technologies across different socioeconomic settings.
- AI integration streamlines preoperative planning by analyzing 3D imaging data (CT/MRI) to create virtual surgical simulations for complex pediatric deformities (e.g., severe scoliosis).
- Predictive modeling powered by machine learning assists orthopedic surgeons in accurately forecasting skeletal maturity and residual growth, crucial for timing guided growth procedures.
- AI algorithms enhance robotic navigation systems, offering real-time intraoperative guidance and improving the precision of screw and pin insertion, particularly near sensitive growth plates.
- The technology facilitates the design and rapid prototyping of patient-specific implants and external fixator components using additive manufacturing (3D printing).
- Large language models (LLMs) and natural language processing (NLP) aid in synthesizing complex pediatric patient data from electronic health records (EHRs) for epidemiological research and treatment protocol development.
- AI-driven image analysis improves the early and accurate detection of subtle fractures, developmental dysplasia of the hip (DDH), and other congenital musculoskeletal disorders.
DRO & Impact Forces Of Ortho Pediatric Devices Market
The dynamics of the Ortho Pediatric Devices Market are fundamentally shaped by the interplay of powerful drivers, stringent regulatory restraints, and expansive opportunities that necessitate precise strategic navigation. The primary market driver is the inherent demand for specialized, growth-accommodating solutions, necessitated by the physiological differences between pediatric and adult skeletal systems, coupled with increasing global awareness and diagnosis rates of congenital and acquired pediatric orthopedic conditions. Technological innovation, particularly in bioresorbable materials and minimally invasive surgical systems designed to minimize disruption to the physis (growth plate), acts as a strong impact force, compelling manufacturers to continuously invest in specialized research and development. Furthermore, supportive governmental initiatives aimed at improving child health and safety standards in developed nations significantly bolster market expansion and product adoption.
Conversely, the market faces considerable restraints, primarily stemming from the relatively low volume of pediatric orthopedic procedures compared to adult reconstructive surgeries, which often results in higher manufacturing costs and extended payback periods for specialized R&D investments. Stringent regulatory pathways, particularly in regions like the US (FDA) and EU (MDR), pose significant challenges, as clinical trials for devices used in children are ethically complex and require long-term follow-up to assess impact on growth. Moreover, the scarcity of pediatric orthopedic specialists globally, particularly in emerging economies, limits the effective adoption and utilization of highly specialized, complex devices, creating a bottleneck in market penetration for advanced technologies and specialized fixation systems.
Opportunities for growth are abundant, centering on the vast, untapped potential within emerging markets, where improvements in healthcare access are steadily creating new demand for quality pediatric care. Significant opportunities also exist in the development of modular and adjustable internal fixation systems that can be customized intraoperatively, reducing inventory burden while ensuring optimal fit for diverse pediatric anatomies. The rising prevalence of sports-related injuries and conditions linked to childhood obesity presents a continuous opportunity for specialized trauma and ligament repair devices. Impact forces are further magnified by the increasing trend toward value-based healthcare, which favors devices and procedures offering superior long-term outcomes, lower revision rates, and minimal inpatient stay, thereby pushing the industry toward greater innovation in areas like non-fusion spinal correction techniques.
Segmentation Analysis
The Ortho Pediatric Devices Market is comprehensively segmented based on product type, specific application, and end-user, providing a granular view of market dynamics and adoption patterns across the pediatric orthopedic care continuum. Analysis reveals that product segmentation is critical, distinguishing between high-volume trauma devices and highly specialized systems like pediatric spinal implants, each demanding different manufacturing precision and regulatory compliance. Application segmentation reflects the varying clinical burden associated with different conditions, ranging from common fractures to complex congenital defects requiring extensive limb reconstruction. Understanding these segments is paramount for manufacturers to align product portfolios with critical clinical needs and for healthcare providers to optimize procurement strategies.
The dominance of specific segments is largely influenced by the burden of disease. For instance, trauma and fracture management remain the largest application segment globally due to the high incidence of pediatric accidents and injuries. However, the fastest growth is observed in the deformity correction segment, specifically for spinal conditions, driven by major technological shifts towards less invasive, dynamic, and growth-friendly solutions. End-user segmentation emphasizes the importance of specialized centers, as complex pediatric cases are typically concentrated in tertiary or quaternary care facilities equipped with the necessary expertise and advanced imaging modalities to safely implement these specialized devices, thereby driving demand in the hospital and specialty clinic sectors.
- By Product Type:
- Internal Fixation Devices (Plates, Screws, Pins, Intramedullary Nails specific to pediatrics)
- External Fixation Systems (Modular, Ring, Hybrid Systems tailored for small anatomies)
- Spinal Systems (Growing Rods, Tethering Systems, Pediatric Pedicle Screws, Fusion Cages)
- Braces and Supports (Custom-molded Orthoses, Off-the-shelf Supports)
- Prosthetics and Orthotics
- By Application:
- Trauma and Fracture Management
- Spinal Deformity Correction (Scoliosis, Kyphosis)
- Limb Length Discrepancy and Deformity Correction
- Sports Injuries and Ligament Repair
- Congenital Deformities (Clubfoot, DDH)
- By End-User:
- Hospitals (Specialty Pediatric and General Orthopedic Departments)
- Specialty Pediatric Orthopedic Clinics
- Ambulatory Surgical Centers (ASCs)
Value Chain Analysis For Ortho Pediatric Devices Market
The value chain for the Ortho Pediatric Devices Market is intricate, characterized by high requirements for specialized material sourcing, precision manufacturing, and highly informed distribution. Upstream activities begin with the procurement of specialized, often high-grade biocompatible materials such as titanium alloys, cobalt-chrome, and advanced polymers (including bioresorbable PLLA/PGA). Research and development holds a disproportionately high weight in the pediatric value chain, focusing heavily on miniaturization, biomechanical testing specific to growing bone, and achieving necessary regulatory clearances. Manufacturers often maintain vertical integration or extremely close collaboration with specialized suppliers to ensure material quality and traceability, which is critical given the stringent safety standards for children's implants. Intellectual property related to growth-sparing technology significantly differentiates key players in the upstream segment.
Midstream activities involve sophisticated, high-precision manufacturing, often incorporating additive manufacturing (3D printing) for creating patient-specific jigs, guides, and complex custom implants, particularly for rare bone defects or severe deformities. Quality control in the midstream is exceptionally rigorous, focusing on sterilization protocols, dimensional accuracy, and ensuring that all devices comply with ISO and regional medical device standards (e.g., MDR compliance). The low volume, high complexity nature of many pediatric devices necessitates flexible manufacturing setups. This segment also includes extensive educational efforts directed at surgeons and clinical staff, often involving cadaver labs and simulation training, which is a mandatory link between manufacturing and adoption.
Downstream activities are dominated by specialized distribution channels. Due to the critical nature and specificity of these devices, sales often rely on direct distribution models or highly trained, dedicated distributors who maintain intimate relationships with pediatric hospitals and surgeons. The distribution channel must manage complex inventory requirements, including consignment stock for emergency trauma cases and rapid logistics for custom-made implants. Direct engagement facilitates critical feedback loops essential for product iteration and improvement. Indirect distribution, though less common for complex implants, is utilized for high-volume, standard products like braces and routine external fixators, often involving group purchasing organizations (GPOs) and hospital procurement networks, emphasizing the need for robust inventory management tailored to specialized needs.
Ortho Pediatric Devices Market Potential Customers
The primary customers in the Ortho Pediatric Devices Market are institutional buyers, specifically large, tertiary care hospitals and specialized pediatric medical centers that manage complex, high-acuity orthopedic cases. These facilities serve as critical nodes due to their concentration of specialized surgeons, advanced imaging technologies (e.g., EOS imaging systems), and multidisciplinary teams necessary for treating conditions like severe scoliosis, congenital limb deficiencies, and polytrauma in children. The purchasing decisions in these institutions are driven not merely by cost, but predominantly by clinical evidence demonstrating superior patient outcomes, minimized risk of revision surgery, device compatibility with long-term growth, and strong supplier support for training and inventory management, making the clinical leadership a crucial target for marketing efforts.
Specialty pediatric orthopedic clinics represent a rapidly growing segment of potential customers. These clinics often handle less complex trauma, common deformities (like clubfoot managed via the Ponseti method), and post-operative follow-up care. While they may not purchase high-end internal fixation systems as frequently as major hospitals, they are significant buyers of external support devices, custom orthotics, casting materials, and basic pediatric trauma kits. The emergence of Ambulatory Surgical Centers (ASCs) focused on minor orthopedic procedures in adolescents, such such as minor fracture fixation or sports injury repair, also presents a distinct customer base demanding standardized, cost-effective pediatric devices and instruments that facilitate quick turnover and reduced inpatient stay.
Ultimately, the end-users—the pediatric orthopedic surgeons, neurosurgeons, and trauma specialists—are the most influential potential customers, driving product selection based on surgical familiarity, device innovation, ease of implantation, and demonstrated safety profiles. Their preferences directly influence the procurement decisions of hospitals and centers. Key decision-makers look for devices that minimize surgical time, reduce radiation exposure during insertion, and offer long-term adaptability. Therefore, manufacturers must target educational and training initiatives directly toward this highly specialized group, establishing credibility and ensuring that the devices meet the unique, evolving requirements of the pediatric musculoskeletal system.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 4,500.0 million |
| Market Forecast in 2033 | USD 8,200.0 million |
| Growth Rate | 9.0% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Orthopediatrics Corp., Pega Medical, WishBone Medical, Inc., Johnson & Johnson (DePuy Synthes), Zimmer Biomet Holdings, Inc., Stryker Corporation, Smith & Nephew plc, Merete Medical, Ti-Med, Vilex, Acumed, Integra LifeSciences, Globus Medical, NuVasive, Medtronic plc, Boston Scientific. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Ortho Pediatric Devices Market Key Technology Landscape
The Ortho Pediatric Devices Market is defined by a technological landscape heavily focused on minimizing intervention, preserving growth potential, and enhancing precision. A cornerstone technology is the adoption of growth-friendly spinal systems, such as magnetically controlled growing rods (MCGRs), which allow for non-invasive spine lengthening via an external remote control, minimizing the need for multiple, high-risk surgical procedures associated with traditional rods. Furthermore, vertebral body tethering (VBT) represents a dynamic correction technique utilizing flexible tethers to guide spinal growth, offering a non-fusion alternative for specific types of scoliosis, thereby preserving mobility and spinal flexibility crucial for young patients. These innovations significantly reduce long-term morbidity and are rapidly replacing older, more rigid fixation methods, establishing a new standard of care in pediatric spinal surgery.
Another crucial technological advancement is the sophisticated utilization of additive manufacturing (3D printing). This technology allows for the rapid creation of patient-specific instrumentation, cutting guides, and complex, custom-designed implants tailored precisely to the unique skeletal anatomy of a child afflicted by rare or severe congenital defects. 3D-printed bioresorbable implants, particularly screws and plates made from polylactic acid (PLA) derivatives, are also transforming the market. These materials offer temporary support before gradually dissolving, eliminating the need for a second surgery to remove hardware once healing is complete, thereby improving patient comfort and reducing healthcare costs and risks associated with repeat procedures.
Finally, advanced imaging and navigation technologies are becoming indispensable. Low-dose radiation imaging systems, such as bi-planar slot-scanning radiography (EOS imaging), provide high-quality 3D models of the pediatric skeleton with significantly reduced radiation exposure, crucial for patients requiring frequent follow-up scans (e.g., scoliosis patients). Simultaneously, robotic and navigation systems, initially developed for adult surgery, are being miniaturized and adapted for pediatric use. These systems enhance the surgeon's ability to accurately place fixation elements in small, delicate bones, particularly around the physis, mitigating the severe risks of iatrogenic growth arrest and ensuring optimal long-term functional alignment for conditions ranging from trauma fixation to intricate limb reconstruction procedures.
Regional Highlights
Regional dynamics within the Ortho Pediatric Devices Market reflect significant disparities in healthcare spending, regulatory maturity, and the availability of specialized surgical expertise. North America, driven primarily by the robust US market, maintains its lead due to sophisticated healthcare infrastructure, high prevalence of complex pediatric orthopedic conditions requiring advanced interventions, and favorable reimbursement policies supporting the use of premium, specialized devices. The presence of leading global manufacturers and a strong focus on clinical research and innovation further solidifies the region's dominant position, particularly in pioneering areas like growth-sparing spinal surgery and personalized 3D-printed orthotics.
Europe represents a mature market with high product adoption, particularly in Western European countries like Germany, the UK, and France. The market here is strongly influenced by centralized healthcare systems and adherence to the Medical Device Regulation (MDR), which enforces high safety and efficacy standards. While growth rates are moderate compared to APAC, continuous investment in specialized pediatric trauma centers and the adoption of cutting-edge technology, especially in bioresorbable materials and minimally invasive techniques, sustain its stability and high value. Eastern Europe offers nascent opportunities as healthcare modernization efforts accelerate and access to specialized pediatric care improves.
The Asia Pacific (APAC) region is forecasted to be the engine of market expansion. This aggressive growth is fueled by a massive, growing patient population, rising disposable incomes leading to higher out-of-pocket spending on quality medical care, and continuous investment by governments and private entities in establishing specialized pediatric hospitals. Countries like China, India, and South Korea are experiencing substantial improvements in healthcare access and diagnosis rates for congenital and acquired musculoskeletal conditions, creating an immense, previously underserved demand. Although regulatory fragmentation remains a challenge, the rising awareness and necessity for pediatric-specific implants, moving away from repurposed adult devices, are the primary catalysts for expansion.
- North America: Dominant market share; driven by advanced infrastructure, high healthcare spending, and rapid adoption of innovative surgical technologies like MCGRs and VBT.
- Europe: Mature market; sustained by stringent quality standards (MDR) and specialization in advanced spine and trauma management within established healthcare systems.
- Asia Pacific (APAC): Highest projected CAGR; expansion fueled by large patient populations, improving economic conditions, and increasing access to specialized pediatric healthcare in emerging economies.
- Latin America (LATAM): Emerging growth market; hindered slightly by economic volatility but supported by improving healthcare access and rising foreign investment in trauma centers.
- Middle East & Africa (MEA): Growing market potential, particularly in GCC countries, driven by medical tourism and significant investments in high-quality healthcare infrastructure targeting complex pediatric procedures.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Ortho Pediatric Devices Market.- Orthopediatrics Corp.
- Pega Medical
- WishBone Medical, Inc.
- Johnson & Johnson (DePuy Synthes)
- Zimmer Biomet Holdings, Inc.
- Stryker Corporation
- Smith & Nephew plc
- Merete Medical GmbH
- Ti-Med, Inc.
- Vilex, Inc.
- Acumed LLC
- Integra LifeSciences Corporation
- Globus Medical, Inc.
- NuVasive, Inc. (now part of Globus Medical)
- Medtronic plc
- Boston Scientific Corporation
- Arthrex, Inc.
- Cook Medical
- Orthofix Medical Inc.
- ConMed Corporation
Frequently Asked Questions
Analyze common user questions about the Ortho Pediatric Devices market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is driving the demand for specialized pediatric orthopedic devices?
Demand is primarily driven by the clinical recognition that adult implants are unsuitable for growing skeletons, increasing incidence of pediatric trauma and congenital disorders (like scoliosis and DDH), and continuous technological advancements in growth-friendly and bioresorbable device materials designed specifically for children.
Which application segment holds the largest market share in pediatric orthopedics?
The Trauma and Fracture Management segment currently holds the largest market share due to the high frequency of pediatric bone injuries. However, the Spinal Deformity Correction segment, driven by innovations like Magnetic Growing Rods (MCGRs) and Vertebral Body Tethering (VBT), is expected to show the highest growth rate during the forecast period.
How is 3D printing impacting the development of pediatric orthopedic devices?
3D printing is fundamentally transforming the market by enabling the rapid production of patient-specific instrumentation and custom implants for complex deformities. This technology ensures optimal fit, reduces surgical time, and enhances overall surgical precision, particularly in complex limb reconstruction cases.
What are the primary restraints affecting the growth of the Ortho Pediatric Devices Market?
Key restraints include the relatively low procedural volume compared to the adult market, which increases manufacturing overhead; high costs associated with specialized research and development; and the ethical complexities and regulatory hurdles involved in obtaining clearance for devices used in vulnerable pediatric populations.
Which region is anticipated to demonstrate the fastest growth in the pediatric devices market?
The Asia Pacific (APAC) region is projected to exhibit the fastest growth, propelled by significant population size, substantial improvements in healthcare infrastructure and accessibility, rising economic prosperity, and the subsequent increase in diagnosis and treatment rates for pediatric musculoskeletal conditions.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager