Procalcitonin Market Size, By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 440164 | Date : Jan, 2026 | Pages : 243 | Region : Global | Publisher : MRU
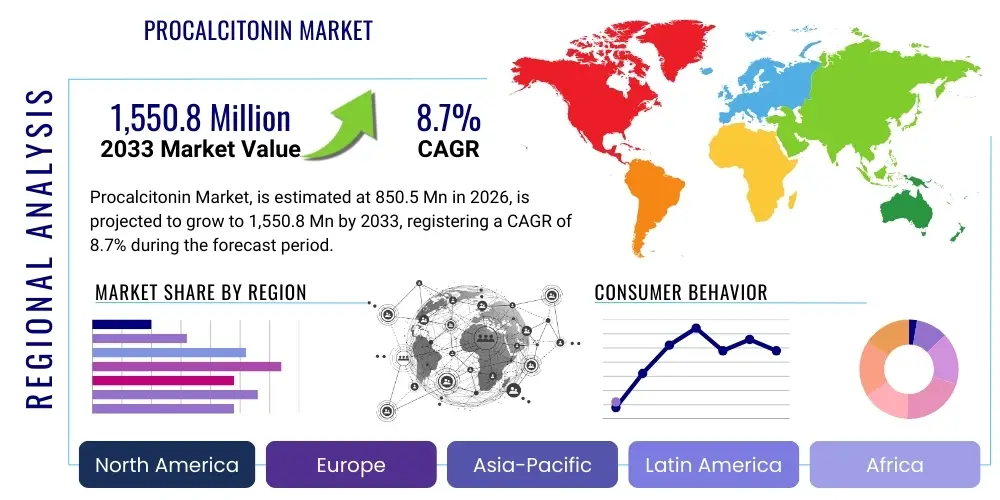
Procalcitonin Market Size
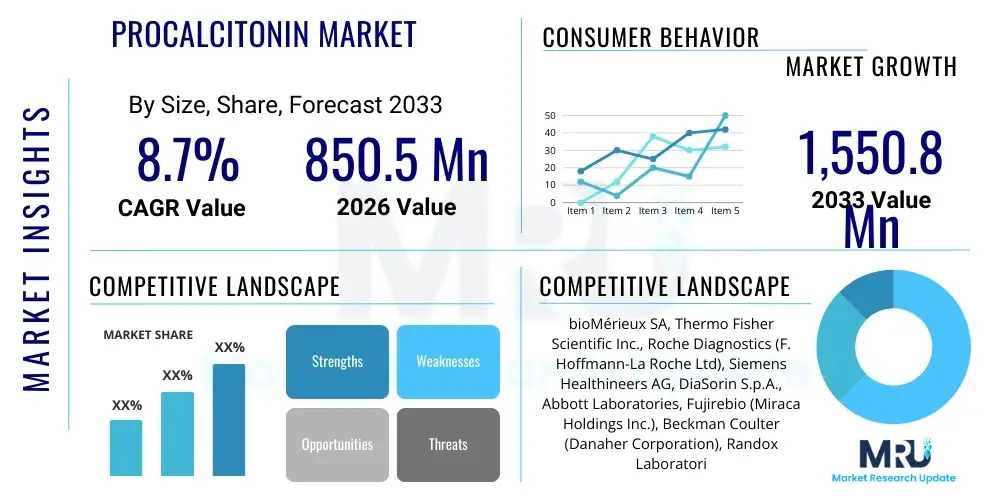
The Procalcitonin Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.7% between 2026 and 2033. The market is estimated at USD 850.5 Million in 2026 and is projected to reach USD 1,550.8 Million by the end of the forecast period in 2033.
Procalcitonin Market introduction
The Procalcitonin (PCT) market encompasses the global ecosystem of diagnostic tests and related services designed to measure procalcitonin levels in human blood serum. Procalcitonin, a prohormone of calcitonin, is recognized as a crucial biomarker for the early diagnosis of bacterial infections, particularly sepsis, a life-threatening condition resulting from the body's overwhelming response to infection. Its concentration in the blood significantly increases during systemic bacterial infections, making it an invaluable tool for differentiating bacterial from viral infections, assessing severity, and guiding antibiotic stewardship programs. The market includes a variety of assay formats, such as automated immunoassays, point-of-care (POC) tests, and semi-quantitative methods, catering to diverse clinical settings from emergency rooms to intensive care units.
Major applications of procalcitonin testing span across various critical care scenarios and infectious disease management. Beyond its primary role in sepsis diagnosis and prognosis, PCT assays are increasingly utilized for guiding antibiotic therapy decisions, helping clinicians determine the appropriate duration of antibiotic treatment, and facilitating early discontinuation of unnecessary antibiotics, thereby combating antimicrobial resistance. The benefits of procalcitonin-guided therapy are substantial, including improved patient outcomes through earlier diagnosis and targeted treatment, reduced hospital stays, and significant cost savings by minimizing excessive antibiotic use. The accuracy and speed of PCT testing contribute to enhanced clinical decision-making, leading to more efficient patient management and resource allocation within healthcare systems globally. This robust diagnostic capability positions procalcitonin as an indispensable tool in modern infectious disease diagnostics.
Driving factors propelling the Procalcitonin market's expansion are multifaceted and deeply rooted in global health challenges. A primary driver is the escalating global incidence of sepsis, coupled with its high mortality and morbidity rates, which underscores the urgent need for rapid and reliable diagnostic markers. The intensifying crisis of antimicrobial resistance (AMR) further amplifies the demand for biomarkers like PCT that can differentiate bacterial infections and guide judicious antibiotic use, thus preserving the efficacy of existing antibiotics. Additionally, the growing awareness among healthcare professionals regarding the clinical utility of PCT, advancements in diagnostic technologies enabling faster and more accurate results, and increasing investments in critical care infrastructure are significant contributors to market growth. The shift towards value-based healthcare models, emphasizing improved patient outcomes and cost-effectiveness, also favors the adoption of procalcitonin testing, which offers tangible benefits in managing complex infectious conditions.
Procalcitonin Market Executive Summary
The Procalcitonin market is experiencing robust growth driven by the escalating global burden of sepsis and the critical need for effective antibiotic stewardship programs. Business trends indicate a strong focus on developing more rapid, accurate, and point-of-care (POC) testing solutions, aiming to integrate PCT assays seamlessly into emergency and critical care workflows. Strategic collaborations between diagnostic companies and healthcare providers, alongside increasing investments in research and development for novel assay technologies, are prominent features of the current market landscape. Furthermore, a rising emphasis on personalized medicine and data-driven clinical decision support systems is fostering innovations in how PCT results are interpreted and applied, moving beyond simple diagnostic indicators to comprehensive prognostic and therapeutic guidance tools. Companies are also expanding their global footprint, particularly in emerging markets where healthcare infrastructure is rapidly developing and the incidence of infectious diseases remains high, presenting significant growth opportunities.
Regional trends reveal North America and Europe as dominant forces, characterized by advanced healthcare systems, high awareness among clinicians, and substantial R&D investments. These regions benefit from established regulatory frameworks that facilitate the adoption of new diagnostic technologies. However, the Asia Pacific region is rapidly emerging as a high-growth market, propelled by increasing healthcare expenditure, a large patient population, improving diagnostic infrastructure, and a rising prevalence of infectious diseases. Countries like China and India are particularly attractive due to their vast populations and increasing medical tourism, leading to greater adoption of advanced diagnostic tools. Latin America, the Middle East, and Africa are also demonstrating growth, albeit at a slower pace, driven by improving access to healthcare and a growing focus on infectious disease management, although challenges related to infrastructure and affordability persist.
Segmentation trends highlight the increasing adoption of automated immunoassays due to their high throughput and precision, although the demand for point-of-care (POC) tests is surging, especially in emergency departments, for rapid decision-making. In terms of application, sepsis diagnosis and management continue to be the primary drivers, but the use of PCT in guiding antibiotic therapy for respiratory tract infections and differentiating systemic bacterial infections from other inflammatory conditions is also gaining traction. End-user segments show hospitals and diagnostic laboratories as the leading consumers, with significant growth anticipated in specialty clinics and physician office laboratories due to the expanding utility of PCT in outpatient settings. The market also observes a trend towards multiplex assays that can simultaneously detect multiple biomarkers, offering a more comprehensive diagnostic picture for complex clinical presentations, further enhancing the value proposition of procalcitonin testing.
AI Impact Analysis on Procalcitonin Market
Users frequently inquire about how artificial intelligence (AI) can enhance the utility and impact of procalcitonin testing in clinical practice. Common questions revolve around AI's ability to improve diagnostic accuracy, facilitate early detection of sepsis, optimize antibiotic treatment strategies, and integrate PCT data with other patient parameters for more comprehensive prognostication. There is a strong interest in AI's potential to overcome the limitations of traditional diagnostic approaches by providing predictive insights, automating data analysis, and supporting evidence-based decision-making. Concerns often touch upon data privacy, the complexity of AI model integration into existing healthcare workflows, and the need for robust validation studies to ensure reliability and clinical relevance. Expectations are high that AI will transform PCT's role from a singular biomarker to an integral component of sophisticated diagnostic and treatment algorithms, leading to more personalized and effective patient care.
- Enhanced Diagnostic Accuracy: AI algorithms can integrate PCT levels with a multitude of other clinical, laboratory, and imaging data, leading to more precise and earlier diagnosis of sepsis and other bacterial infections, surpassing the capabilities of human interpretation alone.
- Predictive Analytics for Sepsis Progression: Machine learning models can analyze patterns in serial PCT measurements and patient vital signs to predict the risk of sepsis progression, organ dysfunction, or mortality, allowing for proactive interventions.
- Optimized Antibiotic Stewardship: AI can develop dynamic treatment guidelines based on individual patient PCT kinetics, recommending optimal antibiotic initiation, duration, and de-escalation, thereby reducing antibiotic misuse and combating antimicrobial resistance more effectively.
- Integration with Electronic Health Records (EHRs): AI systems can seamlessly pull PCT results from laboratory information systems and integrate them into EHRs, providing real-time alerts and decision support tools for clinicians directly within their workflow.
- Personalized Treatment Strategies: By analyzing vast datasets, AI can identify patient subgroups that respond differently to PCT-guided therapy, enabling more personalized and targeted treatment approaches based on individual risk profiles and predicted outcomes.
- Automated Trend Monitoring and Anomaly Detection: AI can continuously monitor PCT levels and automatically detect abnormal trends or rapid changes that might indicate deteriorating patient conditions, prompting immediate clinical review and intervention.
- Support for Research and Development: AI can accelerate the discovery of novel biomarkers and therapeutic targets by identifying complex relationships within large biological datasets, potentially leading to new diagnostic assays or drug development in conjunction with PCT.
DRO & Impact Forces Of Procalcitonin Market
The Procalcitonin market is significantly influenced by a dynamic interplay of drivers, restraints, and opportunities, alongside various impact forces that shape its trajectory. A major driver is the alarming global increase in sepsis incidence and mortality, which necessitates rapid and accurate diagnostic tools like PCT for early intervention. Concurrently, the escalating challenge of antimicrobial resistance (AMR) globally fuels the demand for biomarkers that can guide antibiotic therapy, helping clinicians differentiate between bacterial and viral infections and optimize antibiotic use. This focus on antibiotic stewardship is a powerful catalyst for PCT adoption. Furthermore, continuous advancements in diagnostic technologies, including the development of faster and more sensitive assays and point-of-care testing devices, enhance the accessibility and utility of PCT, driving its market growth. The increasing number of critically ill patients, coupled with expanded critical care infrastructure worldwide, also contributes significantly to the demand for PCT testing.
Despite the strong drivers, several restraints pose challenges to market expansion. The relatively high cost of procalcitonin tests compared to conventional inflammatory markers can be a barrier to widespread adoption, especially in resource-limited settings. Moreover, a lack of comprehensive awareness and standardization in PCT usage guidelines across different healthcare systems and regions can hinder consistent integration into clinical practice. Competition from alternative biomarkers for sepsis and infection, such as C-reactive protein (CRP) or interleukins, also presents a challenge, though PCT often offers superior specificity for bacterial infections. Regulatory complexities and varying reimbursement policies across countries can further impede market entry and growth for new PCT products. The need for specialized equipment and trained personnel for certain advanced PCT assays can also limit their accessibility in some healthcare facilities, particularly in rural or underserved areas.
Opportunities for growth in the Procalcitonin market are abundant and varied. The development and commercialization of advanced point-of-care (POC) PCT testing devices represent a significant opportunity, enabling rapid results at the patient's bedside, particularly in emergency and primary care settings. There is also substantial potential in integrating PCT testing with emerging technologies such as artificial intelligence and machine learning to create sophisticated diagnostic algorithms and predictive models, enhancing clinical decision support. Furthermore, expanding the applications of PCT beyond sepsis, into areas like respiratory tract infections, post-operative complications, and neonatal infections, presents new avenues for market penetration. Emerging economies in Asia Pacific, Latin America, and the Middle East and Africa offer untapped market potential due to their rapidly developing healthcare infrastructures and growing focus on infectious disease management. Strategic partnerships with pharmaceutical companies for companion diagnostics, especially in the context of novel antimicrobial development, also represent a promising area for future growth.
Segmentation Analysis
The Procalcitonin market is intricately segmented to provide a detailed understanding of its various facets, categorized primarily by product type, application, end-user, and technology. This segmentation offers valuable insights into the market dynamics, identifying key growth areas and competitive landscapes within each category. The diversity in product offerings reflects the varying clinical needs and technological advancements, from comprehensive lab-based systems to rapid point-of-care solutions. Applications range from critical care diagnostics to antibiotic stewardship, underscoring the broad utility of procalcitonin as a biomarker. End-user segments illustrate the primary consumers of these diagnostic tools, while technology segmentation highlights the various methodologies employed in PCT detection, each offering distinct advantages in terms of speed, accuracy, and cost.
- By Product Type:
- Reagents & Kits: These include the consumable components necessary for performing PCT assays, such as antibodies, calibrators, and control materials. They represent the largest segment due to recurring purchases.
- Instruments: This segment comprises the automated and semi-automated analyzers used to process PCT assays, ranging from compact benchtop systems to high-throughput laboratory instruments.
- By Application:
- Sepsis Diagnosis: The largest application segment, driven by the critical need for early and accurate detection of sepsis for timely intervention.
- Respiratory Tract Infections: Utilized to differentiate bacterial from viral infections and guide antibiotic therapy in conditions like pneumonia and bronchitis.
- Renal Failure: Used in patients with renal impairment where inflammatory markers might be unreliable, aiding in infection management.
- Other Applications: Includes use in post-operative infections, meningitis, and neonatal sepsis, expanding its utility across various clinical scenarios.
- By End-User:
- Hospitals: The primary end-users, particularly critical care units, emergency departments, and general wards, due to the high volume of critically ill patients and need for rapid diagnostics.
- Diagnostic Laboratories: Centralized labs performing high-volume testing for hospitals, clinics, and outpatient facilities.
- Specialty Clinics: Including intensive care clinics, infectious disease clinics, and pediatric clinics where PCT testing can guide specific patient management.
- Research & Academic Institutes: Utilizing PCT for clinical studies, biomarker discovery, and educational purposes.
- By Technology:
- Immunoassay: The dominant technology, including methods like ELISA (Enzyme-Linked Immunosorbent Assay), Chemiluminescence Immunoassay (CLIA), and Fluorescence Immunoassay (FIA), offering high sensitivity and specificity.
- Point-of-Care (POC) Testing: Rapid diagnostic methods designed for immediate results at or near the patient's location, critical for emergency settings.
- Other Technologies: Encompassing alternative or emerging detection methods, potentially involving molecular diagnostics or microfluidics for PCT measurement.
Value Chain Analysis For Procalcitonin Market
The value chain for the Procalcitonin market is a complex interplay of various stakeholders, starting from raw material suppliers and R&D, moving through manufacturing, and culminating in delivery to end-users. The upstream analysis involves the foundational elements, beginning with the provision of high-quality reagents, antibodies, and biochemical components necessary for PCT assay development. This segment is characterized by specialized chemical and biotechnological companies that supply highly purified procalcitonin antigens, antibodies (monoclonal and polyclonal), and other critical biomolecules. Research and development activities, often conducted by biotechnology firms, academic institutions, and diagnostic companies, form a vital part of the upstream segment, focusing on discovering new assay methodologies, improving test sensitivity and specificity, and exploring novel applications for procalcitonin testing. Intellectual property and patent protection are crucial in this early stage, driving innovation and competitive advantage.
Moving downstream, the value chain encompasses the manufacturing and distribution processes that bring PCT assays to market. Diagnostic companies are central to this stage, undertaking the development, production, and quality control of a wide array of PCT products, including reagents, kits, and instruments. This involves stringent regulatory compliance, adherence to Good Manufacturing Practices (GMP), and comprehensive validation studies to ensure product reliability and safety. The distribution channel then plays a pivotal role in market penetration and accessibility. This often involves a multi-tiered approach, combining both direct and indirect sales strategies. Direct distribution involves manufacturers selling directly to large hospital networks, government health agencies, or major diagnostic laboratory chains, allowing for greater control over sales and customer relationships, as well as providing specialized technical support and training.
Indirect distribution, on the other hand, relies heavily on a network of third-party distributors, wholesalers, and medical supply companies. These intermediaries are crucial for reaching a broader customer base, including smaller hospitals, clinics, and private laboratories, especially in geographically dispersed or emerging markets. These distributors often possess established logistics networks, local market expertise, and existing client relationships, facilitating efficient market penetration. The choice between direct and indirect channels often depends on market maturity, geographical reach, and the specific strategic objectives of the diagnostic company. The final stage of the value chain involves the end-users, such as hospitals (including intensive care units and emergency departments), diagnostic laboratories, and specialty clinics, which utilize PCT tests for patient management, ultimately benefiting patients through improved diagnostic accuracy and targeted treatment. Effective communication and educational initiatives throughout the distribution channels are vital to ensure proper adoption and utilization of PCT diagnostics by healthcare professionals.
Procalcitonin Market Potential Customers
The Procalcitonin market primarily serves a diverse range of end-users and buyers within the healthcare ecosystem, all united by the need for reliable and rapid diagnostic tools to manage infectious diseases and critical conditions. The most significant potential customers are hospitals, encompassing their various departments such as intensive care units (ICUs), emergency departments (EDs), general medical and surgical wards, and infectious disease departments. Within ICUs and EDs, the demand for PCT is particularly high due to the critical nature of patient conditions and the imperative for swift diagnostic decisions to initiate appropriate treatment, especially for suspected sepsis. Hospitals rely on PCT for early diagnosis, prognosis assessment, and guiding antibiotic stewardship programs to optimize patient outcomes and reduce healthcare costs associated with prolonged hospital stays and inappropriate antibiotic use.
Another major segment of potential customers includes diagnostic laboratories, both centralized clinical laboratories and commercial reference laboratories. These facilities perform a high volume of PCT tests for a wide array of referring healthcare providers, including hospitals, outpatient clinics, and physician offices. Diagnostic laboratories require automated, high-throughput systems capable of processing numerous samples efficiently and accurately, contributing to the scalability and standardization of PCT testing across regions. They play a crucial role in providing timely and precise results that inform clinical decisions, often serving as a central hub for diagnostic services within healthcare networks. Their purchasing decisions are often influenced by assay robustness, automation capabilities, cost-per-test, and integration with existing laboratory information systems.
Beyond these primary segments, specialty clinics, such as those focusing on infectious diseases, pulmonology, critical care medicine, and pediatrics, represent a growing segment of potential customers. These clinics utilize PCT testing for more specialized patient management, including monitoring chronic infections, evaluating post-operative complications, and managing specific patient populations like neonates or immunocompromised individuals. Furthermore, research and academic institutions are important customers, purchasing PCT reagents and kits for clinical studies, biomarker research, and the development of new diagnostic applications. With the increasing emphasis on evidence-based medicine and personalized treatment approaches, pharmaceutical companies also engage with PCT diagnostics, particularly in the context of clinical trials for new antimicrobial agents, where PCT can serve as a valuable biomarker for patient stratification or treatment response monitoring.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 850.5 Million |
| Market Forecast in 2033 | USD 1,550.8 Million |
| Growth Rate | 8.7% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | bioMérieux SA, Thermo Fisher Scientific Inc., Roche Diagnostics (F. Hoffmann-La Roche Ltd), Siemens Healthineers AG, DiaSorin S.p.A., Abbott Laboratories, Fujirebio (Miraca Holdings Inc.), Beckman Coulter (Danaher Corporation), Randox Laboratories Ltd., EDAN Instruments, Inc., Mindray Medical International Limited, Snibe Diagnostics, Lansion Biotechnology Co., Ltd., Wondfo Biotech Co., Ltd., Luminex Corporation (A part of DiaSorin Group), BGI Genomics, Bio-Rad Laboratories, Inc., Qiagen N.V., GenMark Diagnostics (A Roche Company), QuidelOrtho Corporation. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Procalcitonin Market Key Technology Landscape
The Procalcitonin market's technology landscape is characterized by a continuous evolution towards greater speed, accuracy, automation, and accessibility in diagnostic testing. Immunoassay techniques remain the cornerstone of PCT measurement, with Chemiluminescence Immunoassay (CLIA) and Fluorescence Immunoassay (FIA) being dominant due to their high sensitivity, broad dynamic range, and compatibility with automated laboratory analyzers. These technologies enable high-throughput processing, crucial for large diagnostic laboratories and hospitals, providing reliable quantitative results quickly. ELISA (Enzyme-Linked Immunosorbent Assay) is also widely used, particularly in research settings and for smaller-scale diagnostic needs, offering robust performance with a lower initial investment compared to fully automated systems. The ongoing refinement in antibody development and labeling techniques continues to enhance the performance characteristics of these immunoassays, improving detection limits and reducing assay interference, thereby leading to more precise and clinically actionable results.
A significant technological trend shaping the market is the rapid advancement in Point-of-Care (POC) testing solutions for procalcitonin. These portable devices are designed to deliver quick results at the patient's bedside, in emergency rooms, or in remote clinical settings, circumventing the need for specialized laboratory infrastructure and reducing turnaround times. POC PCT tests typically employ lateral flow immunoassays or compact automated analyzers, offering qualitative, semi-quantitative, or quantitative results within minutes. The convenience and immediacy of POC testing are critical for conditions like sepsis, where early diagnosis directly impacts patient outcomes. Innovations in microfluidics and miniaturization are further driving the development of more sophisticated and user-friendly POC devices, making PCT testing more accessible and integrated into immediate clinical decision-making processes. The focus for POC platforms is on ease of use, minimal sample volume requirements, and robust performance in non-laboratory environments, pushing the boundaries of decentralized diagnostics.
Beyond traditional immunoassays, the technology landscape is also witnessing the emergence of multiplex assay platforms and integrated diagnostic systems. Multiplex assays enable the simultaneous detection of procalcitonin alongside other relevant biomarkers (e.g., CRP, IL-6) from a single patient sample, providing a more comprehensive diagnostic profile for complex inflammatory and infectious conditions. This approach aids in differential diagnosis and offers a more nuanced understanding of a patient's immune response. Furthermore, there is a growing trend towards integrating PCT testing platforms with hospital information systems (HIS) and laboratory information systems (LIS) to facilitate seamless data management, result reporting, and clinical decision support. This integration enhances workflow efficiency, reduces manual errors, and allows for real-time monitoring of patient data, which is essential for effective antibiotic stewardship programs and critical care management. The convergence of advanced analytical methods with digital health technologies is set to further revolutionize the role and impact of procalcitonin in infectious disease diagnostics.
Regional Highlights
- North America: This region stands as a dominant force in the Procalcitonin market, primarily driven by a highly advanced healthcare infrastructure, significant investments in research and development, and a high awareness among clinicians regarding the importance of rapid sepsis diagnosis and antibiotic stewardship. The United States, in particular, leads in adopting novel diagnostic technologies and benefits from robust reimbursement policies, which further facilitate market growth. The presence of major market players and a high incidence of chronic diseases contributing to sepsis also bolster demand. Canada exhibits steady growth, focusing on enhancing critical care services and implementing national guidelines for infection management.
- Europe: Europe represents another substantial market for procalcitonin, characterized by stringent healthcare guidelines for infection control and antibiotic use, particularly in countries like Germany, France, and the UK. Strong governmental initiatives to combat antimicrobial resistance and significant investments in healthcare diagnostics contribute to market expansion. The region benefits from a well-established network of diagnostic laboratories and hospitals that are keen on adopting advanced biomarkers for improved patient management. However, varying reimbursement policies and economic disparities across member states can influence market penetration.
- Asia Pacific (APAC): The Asia Pacific region is rapidly emerging as the fastest-growing market for procalcitonin. This growth is fueled by increasing healthcare expenditure, a vast and aging population prone to infections, improving diagnostic infrastructure, and a rising awareness of advanced diagnostic techniques. Countries such as China and India are at the forefront of this expansion, driven by their large patient pools, growing medical tourism, and increasing government support for healthcare reforms. The adoption of advanced diagnostics in Japan, South Korea, and Australia also contributes significantly to regional market revenue.
- Latin America: This region is experiencing steady growth in the Procalcitonin market, primarily due to improving healthcare access, increasing investments in critical care facilities, and a rising prevalence of infectious diseases. Countries like Brazil, Mexico, and Argentina are leading the adoption of PCT testing, driven by a growing focus on enhancing diagnostic capabilities and addressing public health challenges related to infections. Economic instabilities and varying healthcare policies, however, can sometimes pose challenges to uniform market development.
- Middle East and Africa (MEA): The MEA region is a nascent but promising market for procalcitonin. Growth is attributed to increasing healthcare spending, modernization of healthcare facilities, and a growing recognition of the burden of infectious diseases, particularly in wealthier Gulf Cooperation Council (GCC) countries. However, challenges such as limited healthcare infrastructure in some sub-Saharan African countries and lower awareness levels hinder broader market penetration. International collaborations and public health initiatives are vital for accelerating market growth in this region.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Procalcitonin Market.- bioMérieux SA
- Thermo Fisher Scientific Inc.
- Roche Diagnostics (F. Hoffmann-La Roche Ltd)
- Siemens Healthineers AG
- DiaSorin S.p.A.
- Abbott Laboratories
- Fujirebio (Miraca Holdings Inc.)
- Beckman Coulter (Danaher Corporation)
- Randox Laboratories Ltd.
- EDAN Instruments, Inc.
- Mindray Medical International Limited
- Snibe Diagnostics
- Lansion Biotechnology Co., Ltd.
- Wondfo Biotech Co., Ltd.
- Luminex Corporation (A part of DiaSorin Group)
- BGI Genomics
- Bio-Rad Laboratories, Inc.
- Qiagen N.V.
- GenMark Diagnostics (A Roche Company)
- QuidelOrtho Corporation
Frequently Asked Questions
What is procalcitonin and why is it important?
Procalcitonin (PCT) is a protein released into the bloodstream in response to systemic bacterial infections, making it a crucial biomarker. It is important because its levels rise rapidly and significantly during bacterial infections, particularly sepsis, enabling early and accurate differentiation from viral infections and guiding appropriate antibiotic therapy, which improves patient outcomes and combats antimicrobial resistance.
How is procalcitonin testing used in clinical practice?
Procalcitonin testing is primarily used for the early diagnosis and prognosis of sepsis and other bacterial infections, especially in critical care settings. It helps clinicians decide whether to initiate or discontinue antibiotics, thereby optimizing antibiotic stewardship, reducing unnecessary antibiotic exposure, and shortening hospital stays, particularly in conditions like respiratory tract infections.
What are the key drivers of the Procalcitonin market growth?
The key drivers for the Procalcitonin market include the increasing global incidence of sepsis, the growing crisis of antimicrobial resistance (AMR), continuous advancements in diagnostic technologies (especially point-of-care testing), and the expanding focus on antibiotic stewardship programs in healthcare systems worldwide.
What technological advancements are impacting the Procalcitonin market?
Technological advancements significantly impacting the PCT market include the development of highly sensitive automated immunoassay systems (CLIA, FIA), miniaturized and rapid point-of-care (POC) testing devices, and the integration of PCT assays with artificial intelligence (AI) and machine learning for enhanced diagnostic accuracy and predictive analytics.
Which regions are leading the adoption of procalcitonin testing?
North America and Europe currently lead the adoption of procalcitonin testing due to their advanced healthcare infrastructures, high clinician awareness, and robust reimbursement policies. However, the Asia Pacific region is rapidly emerging as a significant growth market, driven by increasing healthcare expenditure and a large patient population in countries like China and India.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager