Stem Cell Media Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 435937 | Date : Dec, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Stem Cell Media Market Size
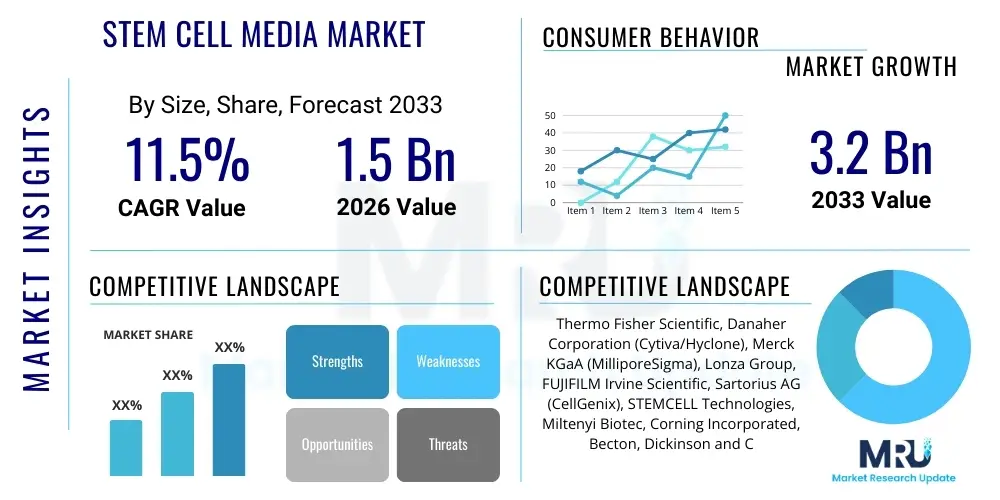
The Stem Cell Media Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 11.5% between 2026 and 2033. The market is estimated at USD 1.5 Billion in 2026 and is projected to reach USD 3.2 Billion by the end of the forecast period in 2033.
Stem Cell Media Market introduction
The Stem Cell Media Market comprises specialized nutrient formulations essential for the in vitro cultivation, maintenance, proliferation, and controlled differentiation of various stem cell populations, including induced pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), and mesenchymal stem cells (MSCs). These complex biological solutions provide the necessary physiological cues, growth factors, vitamins, and minerals required to mimic the natural cellular environment, ensuring cell viability and directed lineage commitment, which are critical for both fundamental research and clinical translation in regenerative medicine. The market is primarily driven by the exponential growth in cell and gene therapy clinical trials, demanding high volumes of clinical-grade, chemically defined, and xeno-free media to minimize variability and comply with stringent regulatory standards. Major applications lie in drug discovery screening, disease modeling, toxicology testing, and large-scale therapeutic manufacturing.
Stem Cell Media Market Executive Summary
The Stem Cell Media Market is characterized by a strong shift toward defined and xeno-free formulations, reflecting heightened regulatory scrutiny and the industry's focus on clinical safety and reproducibility. Business trends indicate aggressive mergers and acquisitions (M&A) among established life science tool providers to capture specialized media technologies, alongside significant investment in automation and large-scale bioreactor technologies which mandate highly optimized media performance. Regionally, North America maintains market dominance due to robust biotechnology infrastructure and heavy R&D expenditure, while the Asia Pacific region is poised for the fastest growth, fueled by government initiatives promoting stem cell research and establishing localized cell therapy manufacturing hubs. Segment trends highlight the increasing prominence of induced Pluripotent Stem Cell (iPSC) media, given their versatility in personalized medicine, and a strong commercial preference for custom media formulations tailored specifically for high-efficiency clinical manufacturing processes under Good Manufacturing Practice (GMP) guidelines.
AI Impact Analysis on Stem Cell Media Market
Common user questions regarding AI's influence in the Stem Cell Media Market focus on its ability to rapidly optimize complex, multi-component formulations, reduce the labor involved in traditional empirical testing, and predict optimal conditions for large-scale cell expansion. Users frequently inquire about the integration of AI platforms with high-throughput screening and automated bioprocessing systems. The key theme is the expectation that AI and Machine Learning (ML) will transition media development from a trial-and-error process to a precise, data-driven design strategy, significantly accelerating the discovery of novel, high-performance growth factors and reducing the overall cost and variability associated with therapeutic cell production. This predictive capability is seen as essential for scaling cell therapy manufacturing globally.
- AI algorithms accelerate the discovery of novel media components and growth factors by analyzing vast omics data (genomics, proteomics, metabolomics) linked to cell performance.
- Machine Learning models optimize media formulations by identifying synergistic interactions between components, predicting the ideal concentration ratios for specific cell types (e.g., neural progenitors vs. cardiac progenitors).
- Predictive analytics enables real-time quality control during manufacturing, allowing automated adjustment of media feeds or environmental parameters within bioreactors to maintain peak cell health and consistency.
- AI facilitates the development of personalized or patient-specific media protocols, correlating genetic profiles with nutrient uptake requirements to maximize therapeutic yield.
- Automation integration driven by AI minimizes human error and reduces batch-to-batch variability, which is critical for achieving clinical-grade reproducibility (GMP compliance).
- Computational modeling allows virtual screening of thousands of potential media compositions, drastically reducing the time and resources needed for physical empirical testing.
DRO & Impact Forces Of Stem Cell Media Market
The market dynamics are primarily propelled by robust investment in regenerative medicine research, the expanding number of FDA-approved and late-stage clinical trials utilizing stem cells, and the crucial regulatory requirement demanding defined, xeno-free media for clinical applications to ensure patient safety and manufacturing consistency. Restraints include the high capital investment required for developing and validating clinical-grade media, supply chain vulnerabilities for specialized, proprietary components, and the inherent biological complexity of optimizing media for diverse and sensitive stem cell types. Opportunities are centered on developing highly specialized, low-cost, and scalable synthetic media substitutes, penetrating emerging Asian markets with increasing cell therapy infrastructure, and leveraging advanced manufacturing technologies like continuous bioprocessing which necessitate novel, high-concentration media formulations. The primary impact forces revolve around rapid technological substitution (moving from serum-containing to defined media) and intensive regulatory harmonization globally.
Segmentation Analysis
The Stem Cell Media Market is highly diversified, segmented across various parameters including product type, application, cell source, and end-user. Understanding these segments is crucial for strategic market positioning, as performance requirements vary significantly between academic research and clinical manufacturing. The shift towards proprietary, defined formulations drives high-value sales in the Product Type segment, while regenerative medicine and therapeutic manufacturing dominate the Application landscape due to the sheer volume requirements and premium pricing associated with clinical-grade products. Induced Pluripotent Stem Cells (iPSCs) are rapidly gaining market share within the Cell Source segment, reflecting their promise in personalized medicine and disease modeling, creating a high demand for specific iPSC maintenance and differentiation kits.
- By Product Type:
- Defined Media (Serum-Free, Chemically Defined, Xeno-Free)
- Classical Media
- Specialized Media (Custom/Tailored Formulations)
- Supplements and Reagents (Growth Factors, Cytokines)
- By Cell Source:
- Induced Pluripotent Stem Cells (iPSCs)
- Mesenchymal Stem Cells (MSCs)
- Hematopoietic Stem Cells (HSCs)
- Embryonic Stem Cells (ESCs)
- Neural Stem Cells (NSCs)
- By Application:
- Regenerative Medicine and Therapy Development
- Drug Discovery and Toxicology Testing
- Research Applications (Disease Modeling, Basic Biology)
- By End User:
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Contract Research Organizations (CROs) & Cell Banks
Value Chain Analysis For Stem Cell Media Market
The value chain for stem cell media is complex, beginning with the upstream sourcing and manufacturing of highly specialized raw materials, primarily recombinant proteins, growth factors, proprietary peptides, and high-purity basal salts. Upstream analysis reveals intense reliance on a few key suppliers for high-grade, GMP-compliant components, leading to potential supply chain bottlenecks and high input costs. Specialized media manufacturers then formulate, test, and package these components, adhering to rigorous quality standards and often providing proprietary protocols. Distribution channels involve both direct sales, particularly for large clinical-stage clients who require custom logistics and technical support, and indirect sales through specialized global and regional distributors who handle logistics for academic and smaller research institutions. Downstream activities involve end-user consumption in high-throughput research laboratories and large-scale biomanufacturing facilities, where performance, scalability, and technical support dictate procurement decisions. Direct engagement allows manufacturers to gather crucial feedback for iterative product improvement and optimization for bioprocessing needs.
Stem Cell Media Market Potential Customers
The primary consumers and buyers of stem cell media are institutions heavily invested in cell biology and translational research. Pharmaceutical and biotechnology companies are the largest segment, driven by their extensive pipelines in cell and gene therapy manufacturing, requiring high-volume, GMP-grade media for clinical trials and commercial production. Academic and government-funded research institutes constitute another major customer base, focusing on fundamental research, disease mechanism elucidation, and early-stage drug screening. Additionally, specialized contract research organizations (CROs) and Contract Development and Manufacturing Organizations (CDMOs) represent rapidly growing buyers, as they manage the outsourced production and testing of cell therapies for their clients. Cell banks and tissue engineering centers also depend on standardized media for long-term storage and expansion protocols.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 1.5 Billion |
| Market Forecast in 2033 | USD 3.2 Billion |
| Growth Rate | 11.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Thermo Fisher Scientific, Danaher Corporation (Cytiva/Hyclone), Merck KGaA (MilliporeSigma), Lonza Group, FUJIFILM Irvine Scientific, Sartorius AG (CellGenix), STEMCELL Technologies, Miltenyi Biotec, Corning Incorporated, Becton, Dickinson and Company (BD), Bio-Rad Laboratories, Takara Bio, PromoCell GmbH, General Electric (GE Healthcare), R&D Systems (Bio-Techne), ATCC, Cell Applications Inc., PeproTech (Bio-Techne), Sino Biological, HiMedia Laboratories. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Stem Cell Media Market Key Technology Landscape
The key technological advancements shaping the stem cell media market revolve around the move toward chemically defined, xeno-free, and animal-component-free (ACF) formulations. These media minimize safety risks, reduce immunological complications in therapeutic applications, and ensure regulatory compliance necessary for GMP manufacturing. Significant research is being dedicated to developing novel synthetic matrices and hydrogels that can be integrated into the media, providing three-dimensional culture environments that enhance cell functionality and expansion yield, particularly for complex tissues like organoids and spheroids. High-throughput screening (HTS) coupled with automated liquid handling systems is now standard for rapidly testing thousands of media component combinations, dramatically accelerating the optimization process. Furthermore, microfluidic technology is emerging as a critical tool for developing patient-specific media and conducting small-volume, highly controlled differentiation experiments, paving the way for personalized regenerative medicine protocols.
Regional Highlights
The global Stem Cell Media market exhibits significant regional variation in terms of adoption rates, research intensity, and regulatory environment. North America currently holds the largest market share, predominantly driven by the United States. This dominance is attributed to substantial government and private sector funding directed towards life sciences research, the presence of major pharmaceutical and biotechnology hubs, and a well-established regulatory pathway (FDA) that encourages the commercialization of cell and gene therapies. High adoption rates of advanced bioprocessing technologies and the concentration of key market players who pioneer defined media formulations further solidify this region's leadership. The stringent demand for GMP compliance in clinical manufacturing in the US ensures continued high-value consumption of premium, clinical-grade media.
Europe represents the second-largest market, characterized by strong academic research output, significant investments through organizations like the European Medicines Agency (EMA), and established consortia focused on regenerative medicine translation. Key countries such as Germany, the UK, and France are driving demand through clinical trials, particularly those involving CAR T-cell therapies and neurological disorder research. European regulatory focus often emphasizes ethical sourcing and xeno-free media, leading to early adoption of defined formulations. However, fragmentation across national healthcare systems presents certain challenges in rapid commercial scaling compared to the highly centralized North American market.
Asia Pacific (APAC) is projected to be the fastest-growing region during the forecast period. This rapid expansion is fueled by increasing government initiatives in China, South Korea, and Japan to establish domestic cell therapy manufacturing capabilities and dedicated regenerative medicine research parks. Growing healthcare expenditure, the establishment of favorable regulatory frameworks (e.g., Japan's expedited approval system for regenerative therapies), and a rising patient population needing advanced treatments are accelerating market penetration. Localized media manufacturing and strategic collaborations between international market leaders and regional academic institutions are defining the competitive landscape in APAC, targeting high-volume production at competitive pricing points.
- North America (Dominant Market): High R&D spending, extensive presence of leading biotech companies, and robust regulatory framework facilitating clinical translation. Focus on personalized medicine and high-throughput screening technologies.
- Europe (Second Largest Market): Strong academic research base, increasing clinical trial volume in oncology and cardiology, driven by stringent quality requirements favoring defined media solutions.
- Asia Pacific (Fastest Growing Market): Government support for biomedical science (especially in China and Japan), growing infrastructure for cell therapy manufacturing, and increasing strategic partnerships focusing on localized, scalable production.
- Latin America & MEA: Emerging markets characterized by increasing awareness and initial investments in stem cell banking and localized research projects, albeit limited by funding constraints and less mature regulatory environments compared to developed economies.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Stem Cell Media Market.- Thermo Fisher Scientific Inc.
- Danaher Corporation (Cytiva/Hyclone)
- Merck KGaA (MilliporeSigma)
- Lonza Group
- FUJIFILM Irvine Scientific
- STEMCELL Technologies Inc.
- Sartorius AG (CellGenix)
- Corning Incorporated
- Miltenyi Biotec B.V. & Co. KG
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories, Inc.
- Takara Bio Inc.
- PromoCell GmbH
- General Electric (GE Healthcare)
- R&D Systems (Bio-Techne)
- ATCC
- Cell Applications Inc.
- Sino Biological Inc.
- HiMedia Laboratories Pvt. Ltd.
- PeproTech (Bio-Techne)
Frequently Asked Questions
Analyze common user questions about the Stem Cell Media market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between classical and defined stem cell media?
Classical media typically contain fetal bovine serum (FBS), introducing high variability and contamination risks. Defined media are formulated using precise, known concentrations of synthetic components, eliminating animal-derived products (xeno-free) to ensure batch consistency and meet strict clinical regulatory standards (GMP).
Why is xeno-free media critical for commercial cell therapy manufacturing?
Xeno-free media eliminates the risk of introducing animal pathogens and immunogenic components (such as non-human sialic acids) into therapeutic products. This regulatory necessity enhances patient safety, reduces variability, and simplifies the path to global regulatory approvals for commercial-scale cell therapies.
Which stem cell type segment is currently driving the highest market revenue?
The Mesenchymal Stem Cell (MSC) media segment currently holds significant revenue due to the high volume of ongoing clinical trials utilizing MSCs for immunomodulation and regenerative repair. However, the Induced Pluripotent Stem Cell (iPSC) segment is projected to exhibit the fastest growth rate.
How does automation impact the future development of stem cell media?
Automation, particularly high-throughput screening and robotics, allows researchers to test complex media formulations rapidly and systematically, identifying optimal components. This speeds up product development and ensures the scalability and quality control necessary for advanced biomanufacturing.
Which geographical region is expected to experience the highest growth in media demand?
The Asia Pacific (APAC) region, specifically driven by countries like China and Japan, is forecasted to show the highest Compound Annual Growth Rate (CAGR) due to significant national investments in establishing domestic regenerative medicine infrastructure and expanding clinical research capabilities.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
