Treatment-Resistant Hypertension Management Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 435156 | Date : Dec, 2025 | Pages : 246 | Region : Global | Publisher : MRU
Treatment-Resistant Hypertension Management Market Size
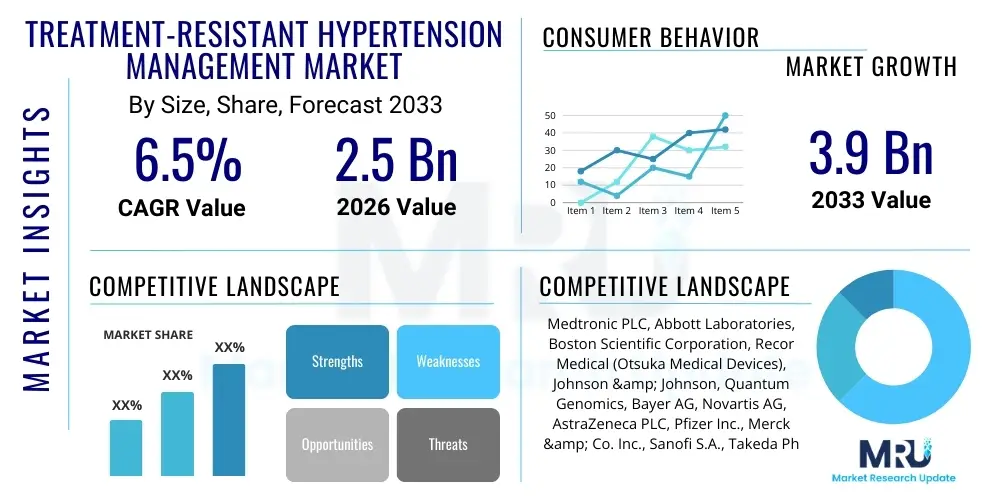
The Treatment-Resistant Hypertension Management Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.5% between 2026 and 2033. The market is estimated at USD 2.5 Billion in 2026 and is projected to reach USD 3.9 Billion by the end of the forecast period in 2033.
Treatment-Resistant Hypertension Management Market introduction
Treatment-Resistant Hypertension (TRH) is clinically defined as the failure to reach the target blood pressure goal despite concurrent use of three antihypertensive agents, including a diuretic, optimally dosed. The management market encompasses pharmacological solutions, primarily novel drug combinations and repositioned therapies, alongside device-based interventions such as Renal Denervation (RDN) and baroreflex activation therapy (BAT). This critical market addresses a significant unmet medical need, as TRH patients face substantially elevated risks of stroke, myocardial infarction, renal failure, and overall cardiovascular mortality. The complexity of TRH pathophysiology, which often involves sympathetic nervous system overactivity, volume overload, and secondary causes like primary aldosteronism, necessitates advanced diagnostic and therapeutic strategies that move beyond standard primary care protocols.
The primary applications of TRH management tools are focused within specialty cardiology clinics, nephrology centers, and dedicated hypertension management units. Pharmacological interventions are advancing beyond traditional calcium channel blockers and renin-angiotensin system inhibitors, incorporating new mineralocorticoid receptor antagonists (MRAs) and endothelin receptor antagonists. Device-based therapies, particularly the resurgence of RDN technologies following positive long-term trial data, represent a paradigm shift, offering non-pharmacological alternatives for patients struggling with medication adherence or those who achieve suboptimal control with polypharmacy. The benefits derived from effective TRH management include reduced long-term cardiovascular burden, improved quality of life for patients, and decreased healthcare expenditures associated with managing TRH-related complications.
Driving factors for the market expansion include the rising global prevalence of hypertension, particularly coupled with increasing rates of obesity and diabetes, which are key risk factors for developing resistance. Furthermore, enhanced clinical awareness among specialists regarding the importance of accurately diagnosing and aggressively treating TRH is crucial. Technological advancements in minimally invasive procedures, coupled with significant investment in clinical trials validating the long-term efficacy and safety of devices like RDN systems, are accelerating market adoption. Regulatory approvals in major economies, specifically the United States and Europe, for next-generation RDN devices are expected to further catalyze growth, positioning device therapy as a legitimate option for patients who remain resistant despite maximizing medical therapy.
Treatment-Resistant Hypertension Management Market Executive Summary
The Treatment-Resistant Hypertension Management market is experiencing a significant resurgence driven primarily by the maturation of device-based therapies and the introduction of novel pharmacological agents targeting underlying mechanisms such as sympathetic activation and mineralocorticoid excess. Key business trends reflect a strategic shift by major medical device companies towards securing regulatory approval for Renal Denervation (RDN) systems, transforming them from investigational products into commercial assets. This shift necessitates substantial investments in physician training and infrastructure development in hospitals to support high procedural volume. Simultaneously, pharmaceutical companies are focusing on synergistic drug combinations and targeted therapies, often leveraging digital health solutions for enhanced patient monitoring and adherence, thereby optimizing therapeutic outcomes and demonstrating value to payers.
Regional trends indicate North America currently holds the largest market share, characterized by high healthcare expenditure, sophisticated diagnostic infrastructure, and rapid uptake of innovative device technologies following landmark clinical trial outcomes. However, the Asia Pacific (APAC) region is projected to exhibit the highest Compound Annual Growth Rate (CAGR) during the forecast period. This accelerated growth in APAC is fueled by the vast, underserved patient pool, improving healthcare access, and increasing penetration of Western-standard treatment protocols in emerging economies like China and India. Europe maintains a steady growth rate, supported by established reimbursement pathways for pharmacological treatments and early adoption of RDN technologies in specialized centers across Germany, the UK, and France.
Segment trends highlight the Device-Based Therapy segment as the fastest growing, particularly within RDN, due to its minimally invasive nature and sustained efficacy demonstrated in long-term studies. Within the pharmacological segment, mineralocorticoid receptor antagonists (MRAs) and novel dual-acting agents are witnessing increased prescriptions, replacing less effective or less tolerated traditional combinations. Furthermore, the Hospital end-user segment dominates revenue generation due to the specialized nature of RDN procedures and the necessity for inpatient or sophisticated outpatient monitoring following complex pharmacological adjustments. Overall market dynamics suggest a future where individualized, mechanism-based combination therapy—integrating pharmacology and device intervention—will become the standard of care for TRH patients, optimizing blood pressure control and minimizing side effects.
AI Impact Analysis on Treatment-Resistant Hypertension Management Market
User queries regarding the impact of Artificial Intelligence (AI) on Treatment-Resistant Hypertension (TRH) management frequently center on themes such as improving diagnostic accuracy, predicting treatment response, and optimizing drug regimens. Users are particularly interested in whether AI can accurately differentiate true resistance from pseudo-resistance (such as white-coat effect or non-adherence), which is a major clinical challenge. Concerns often revolve around the security and interpretability of patient data used in predictive models, and the integration challenges faced by existing clinical workflows when adopting complex algorithms. Expectations are high regarding AI’s potential to personalize therapy, predicting which patients will respond best to specific pharmacological agents or to invasive procedures like Renal Denervation (RDN), thereby significantly reducing costs and clinical inertia associated with trial-and-error treatment approaches. The consensus suggests AI will transition from a supplementary tool to an essential element in complex TRH decision support systems within the next five to seven years.
The application of AI and Machine Learning (ML) algorithms is set to revolutionize the clinical approach to TRH. In diagnostics, AI can analyze large datasets from continuous blood pressure monitoring (CBPM), electronic health records (EHRs), and patient genomics to identify subtle patterns indicative of underlying TRH etiologies, such as high aldosterone levels or severe sympathetic overactivity, often missed by manual review. This leads to faster, more accurate identification of patients who meet strict TRH criteria. Furthermore, AI models are being trained to assess medication adherence based on prescription refill data and patient reported outcomes, helping clinicians distinguish genuine biological resistance from resistance caused by poor compliance.
In treatment planning, predictive analytics powered by AI will allow clinicians to stratify patients based on their likelihood of success with specific treatments. For example, ML models can utilize demographic, physiological, and imaging data (e.g., kidney anatomy) to predict the procedural success and sustained efficacy of RDN, guiding interventional decisions. This personalized approach minimizes unnecessary procedures and avoids prolonged use of ineffective polypharmacy. AI will also play a critical role in optimizing complex drug combinations by analyzing pharmacokinetic interactions and potential side effects, suggesting the most efficacious and safe regimen for each individual TRH patient, ultimately improving long-term therapeutic control and reducing adverse drug events.
- AI enhances diagnostic precision by differentiating true TRH from pseudo-resistance using complex hemodynamic data analysis.
- Machine learning predicts patient responsiveness to specific interventions, such as novel drugs or Renal Denervation (RDN).
- AI algorithms facilitate personalized drug combination optimization, minimizing polypharmacy side effects and improving adherence.
- Integration of AI into continuous blood pressure monitoring (CBPM) allows for real-time risk stratification and early detection of blood pressure spikes.
- Natural Language Processing (NLP) extracts and summarizes relevant clinical data from unstructured electronic health records (EHRs) for faster decision-making in complex cases.
DRO & Impact Forces Of Treatment-Resistant Hypertension Management Market
The dynamics of the Treatment-Resistant Hypertension (TRH) market are shaped by powerful Drivers and Restraints, creating significant Opportunities that collectively constitute the key Impact Forces. The primary driver is the undeniable and increasing burden of uncontrolled hypertension globally, which translates directly into a higher incidence of patients progressing to the resistant stage, requiring specialized and high-cost management. Technological innovation, particularly the clinical validation and increasing regulatory approval of minimally invasive device therapies like Renal Denervation (RDN), serves as a robust opportunity, attracting significant investment and clinical interest. Conversely, major restraints include the high cost associated with advanced diagnostic procedures and device therapies, alongside the inherent complexity of TRH diagnosis, which often leads to misclassification or delayed specialist referral, hindering early market penetration.
The impact forces operate on multiple levels. The positive clinical data emerging from long-term RDN trials (e.g., Symplicity Spyral, Radiance) have created a powerful impact force, shifting physician perception and boosting confidence in device utility. This driver is counterbalanced by the restraint of varying reimbursement policies across global markets, particularly in Asia Pacific and Latin America, which limit patient access to costly procedures. Furthermore, patient non-adherence to complex, multi-drug pharmacological regimens remains a persistent restraint, pushing demand toward device-based solutions that offer greater therapeutic consistency independent of daily patient action. The opportunity lies in leveraging digital health platforms and AI tools to overcome adherence issues, simultaneously strengthening the market for both pharmaceutical and device segments by ensuring optimized treatment delivery and better outcome measurement.
An additional substantial driver is the growing geriatric population, which inherently faces higher rates of multi-morbidity and complex hypertension requiring specialized management. This demographic shift provides a predictable and sustained increase in the patient pool. However, a major restraint is the stringent regulatory scrutiny placed on novel hypertension devices, requiring extensive, long-duration safety and efficacy trials, which slows down product time-to-market. The significant opportunity, therefore, lies in developing combination therapies—integrating low-dose, synergistic pharmacological agents with device-based interventions—to achieve optimal blood pressure targets more effectively than monotherapy or standard polypharmacy, capitalizing on the unmet need for robust, sustained blood pressure reduction in this challenging patient population.
Segmentation Analysis
The Treatment-Resistant Hypertension Management market is comprehensively segmented based on the type of treatment modality, the mechanism of action for pharmacological agents, and the primary end-users receiving these complex services. This segmentation allows for granular analysis of market trends, highlighting the fastest-growing therapeutic areas and identifying critical demographic or institutional segments driving revenue. The primary segmentation distinguishes between Pharmacological Management (drugs) and Device-Based Management (interventional procedures), reflecting the two core therapeutic approaches. Further sub-segmentation within pharmaceuticals focuses on specific drug classes that are effective in TRH, while device segmentation concentrates on specific interventional techniques like RDN and BAT.
- By Treatment Type:
- Pharmacological Management (Drug Therapy)
- Device-Based Management (Interventional Therapy)
- By Drug Class:
- Mineralocorticoid Receptor Antagonists (MRAs) (e.g., Spironolactone, Eplerenone)
- Alpha-Blockers
- Vasodilators
- Other Novel Agents (e.g., Endothelin Receptor Antagonists)
- By Device Type:
- Renal Denervation (RDN) Systems
- Baroreflex Activation Therapy (BAT) Systems
- Carotid Sinus Stimulators
- By End-User:
- Hospitals and Cardiology Centers
- Specialty Clinics
- Ambulatory Surgical Centers (ASCs)
Value Chain Analysis For Treatment-Resistant Hypertension Management Market
The value chain for the Treatment-Resistant Hypertension (TRH) management market is characterized by distinct stages involving specialized inputs, complex manufacturing, highly regulated distribution, and sophisticated end-user delivery. The upstream segment involves intensive research and development (R&D), where pharmaceutical and medtech companies invest heavily in preclinical studies, clinical trials, and regulatory submissions for novel drugs or devices. Key upstream suppliers include API manufacturers for pharmaceuticals (for drug-based solutions) and specialized component manufacturers (e.g., catheter materials, radiofrequency generators, and implantable electronics) for device-based solutions. Quality assurance, intellectual property protection, and stringent regulatory compliance (FDA, EMA) are the central activities defining this stage, necessitating deep expertise and high capital expenditure to secure market entry.
The midstream phase focuses on manufacturing and distribution. Manufacturing processes for TRH drugs involve large-scale chemical synthesis and sterile packaging, while device manufacturing requires precision engineering, assembly of complex electromechanical components, and rigorous sterilization. Distribution channels are specialized due to the high-value, temperature-sensitive nature of some pharmaceuticals and the need for controlled logistics for sterile medical devices. Direct channels are often utilized for device sales, involving direct contracts between the manufacturer and the tertiary care hospital or specialized cardiology center, where the devices are used. Indirect channels, involving wholesalers and specialized medical distributors, are more common for routine pharmacological agents, ensuring broad availability across pharmacies and primary care settings.
The downstream segment encompasses the healthcare providers (Hospitals, Specialty Clinics) and the ultimate patient use. Hospitals and specialized cardiology centers are critical downstream components, as they possess the necessary infrastructure (e.g., catheterization labs, trained interventional cardiologists) required for performing RDN or implanting BAT devices. Diagnostics—particularly Ambulatory Blood Pressure Monitoring (ABPM) and screening for secondary causes—are integrated into the downstream clinical pathway. The value delivery to the patient is highly dependent on physician expertise, effective procedure utilization, and long-term follow-up care. Payers and reimbursement bodies significantly influence the value chain by determining access and utilization rates, especially for high-cost, cutting-edge therapies, thus connecting the final delivery back to upstream R&D justifications.
Treatment-Resistant Hypertension Management Market Potential Customers
The primary potential customers and end-users in the Treatment-Resistant Hypertension (TRH) Management Market are complex healthcare institutions that specialize in chronic disease management and cardiovascular interventions. Hospitals, particularly large tertiary care centers and academic medical institutions, represent the most significant buyer segment. These institutions purchase and utilize the full spectrum of TRH solutions, including high-volume pharmacological agents, advanced diagnostic equipment (like continuous monitoring systems), and high-value interventional devices (like RDN catheters and generators). Their purchasing decisions are driven by the need to manage severe patient populations, improve quality metrics, and justify substantial capital equipment investments through high procedural volumes. They require robust training and technical support services from manufacturers due to the complexity of the procedures involved.
Specialty Clinics, specifically those focused on cardiology, nephrology, and endocrinology, form another critical customer base. These clinics are often referral centers for patients whose hypertension is proving difficult to manage in primary care settings. While these clinics may not perform invasive procedures like RDN directly, they are significant buyers of advanced pharmacological treatments, specialized diagnostic services (such as sophisticated labs for hormonal testing), and digital health solutions designed for remote TRH monitoring and adherence management. Their focus is on optimized medical management and patient stratification, deciding which patients require high-level intervention.
A growing segment of potential customers includes Ambulatory Surgical Centers (ASCs) and dedicated Hypertension Centers of Excellence. As device technologies like RDN become less invasive and potentially shift toward outpatient settings, ASCs are expected to increase their purchasing of RDN systems and related procedural supplies. Furthermore, government health programs and large integrated healthcare networks (IHNs) act as crucial indirect customers. While not direct purchasers of every drug or device, their formulary decisions, treatment guidelines, and reimbursement policies effectively dictate which products are accessible to the broad patient population, thereby steering the procurement patterns of hospitals and clinics across regional markets.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 2.5 Billion |
| Market Forecast in 2033 | USD 3.9 Billion |
| Growth Rate | CAGR 6.5% |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, Recor Medical (Otsuka Medical Devices), Johnson & Johnson, Quantum Genomics, Bayer AG, Novartis AG, AstraZeneca PLC, Pfizer Inc., Merck & Co. Inc., Sanofi S.A., Takeda Pharmaceutical Company Limited, Daiichi Sankyo Company Limited, Boehringher Ingelheim International GmbH, Servier Laboratories, Bristol Myers Squibb, Gilead Sciences, Amgen Inc., Cardiosonic Ltd. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Treatment-Resistant Hypertension Management Market Key Technology Landscape
The technology landscape in the Treatment-Resistant Hypertension (TRH) management market is rapidly evolving, moving beyond conventional pharmaceuticals toward sophisticated, minimally invasive device technologies and advanced digital diagnostics. The most disruptive technology is Renal Denervation (RDN), which uses catheter-based systems (employing radiofrequency ablation or ultrasound energy) to selectively ablate the hyperactive sympathetic nerves in the renal arteries. This technology requires highly specialized catheters and energy generators. The latest RDN systems are designed for increased precision, efficiency, and safety, often featuring improved visualization tools and techniques that minimize procedural time and enhance the durability of the sympathetic nerve effect. The renewed commercial viability of RDN systems, driven by compelling long-term clinical data, is setting a new technological standard for interventional management.
Another crucial technological area is Baroreflex Activation Therapy (BAT). BAT involves the surgical implantation of a small device (similar to a pacemaker) that stimulates the baroreceptors located in the carotid artery, modulating sympathetic and parasympathetic activity to lower blood pressure. This technology targets the autonomic nervous system central to TRH pathogenesis. While RDN is minimally invasive and performed once, BAT is an implantable, ongoing neuromodulation therapy requiring battery replacement over time. The technological advancements in BAT focus on minimizing the size of the implanted generator, improving battery life, and enhancing programmability to optimize individual patient responses. Both RDN and BAT represent significant capital expenditure for healthcare systems but offer a non-pharmacological, long-term therapeutic effect.
Beyond interventional devices, technology advancements in diagnostics and drug delivery are also vital. Continuous and Ambulatory Blood Pressure Monitoring (ABPM) systems are leveraging connectivity (IoT) and miniaturization, enabling more accurate and long-term blood pressure profiling crucial for diagnosing true TRH and assessing treatment efficacy outside the clinic. Furthermore, research in targeted drug delivery systems and novel pharmacological pipelines focuses on compounds that specifically target underlying TRH mechanisms, such as aldosterone synthase inhibition or new classes of selective mineralocorticoid receptor modulators (sMRMs). The integration of these digital diagnostic platforms with AI-driven analytics is the future technological frontier, enabling predictive modeling for personalized TRH management.
Regional Highlights
Regional dynamics in the Treatment-Resistant Hypertension (TRH) Management Market are highly stratified by healthcare expenditure, regulatory speed, and patient awareness of advanced treatment options. North America consistently holds the dominant market share, primarily due to the high prevalence of cardiovascular disease, sophisticated diagnostic infrastructure, and aggressive adoption of high-value interventional devices. The US market, in particular, benefits from favorable reimbursement for specialized procedures and a high volume of specialist referrals. Key growth drivers in this region include the increasing incidence of obesity and diabetes, which accelerate the progression to TRH, and the recent US FDA approval for key RDN systems, significantly bolstering the Device-Based Management segment.
Europe represents the second-largest market, characterized by strong clinical expertise in specialized hypertension centers and generally established (though varied by country) reimbursement pathways for both pharmacological and device therapies. Countries such as Germany, the UK, and France are early adopters of RDN technology, leveraging their strong academic research bases. However, cost containment pressures and centralized purchasing negotiations across some European Union member states can slightly temper market growth compared to the US. The market in Europe is driven by robust clinical guidelines emphasizing rigorous diagnosis and treatment protocols for severe hypertension.
Asia Pacific (APAC) is projected to be the fastest-growing region throughout the forecast period. This rapid expansion is fueled by the vast population base, increasing access to advanced medical care, and rising healthcare spending in major emerging economies like China and India. While device penetration is currently lower due to regulatory lag and infrastructure challenges, the sheer volume of hypertensive patients necessitates innovative management strategies. Market growth in APAC will be concentrated in urban centers where specialist care is available, focusing initially on increasing pharmacological market share and subsequently adopting cost-effective RDN systems as local regulatory approvals are granted. Latin America and the Middle East & Africa (MEA) remain smaller markets, constrained by limited healthcare access and economic factors, but offer future growth potential contingent upon infrastructure modernization and improved funding for chronic disease management.
- North America: Market leader, high adoption of RDN and BAT devices; driven by high TRH prevalence and favorable specialist reimbursement structure.
- Europe: Second-largest market; strong regulatory framework and adoption rates in Western Europe; driven by established hypertension centers and evidence-based protocols.
- Asia Pacific (APAC): Highest CAGR; tremendous untapped patient pool; driven by urbanization, rising middle-class healthcare expenditure, and increasing penetration of advanced therapies in countries like China and Japan.
- Latin America & MEA: Emerging markets; growth potential linked to healthcare infrastructure development and increased government focus on non-communicable diseases.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Treatment-Resistant Hypertension Management Market.- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corporation
- Recor Medical (Otsuka Medical Devices)
- Johnson & Johnson (J&J)
- Quantum Genomics
- Bayer AG
- Novartis AG
- AstraZeneca PLC
- Pfizer Inc.
- Merck & Co. Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Daiichi Sankyo Company Limited
- Boehringher Ingelheim International GmbH
- Servier Laboratories
- Bristol Myers Squibb
- Gilead Sciences
- Amgen Inc.
- Cardiosonic Ltd.
Frequently Asked Questions
Analyze common user questions about the Treatment-Resistant Hypertension management market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary difference between standard hypertension and Treatment-Resistant Hypertension (TRH)?
TRH is defined as blood pressure that remains above the patient's goal despite the optimal use of three antihypertensive medications from different classes, including a diuretic. Standard hypertension typically achieves control with one or two medications, whereas TRH requires complex, often multi-mechanism therapeutic strategies and specialist referral to rule out secondary causes.
How significant is Renal Denervation (RDN) in the future treatment paradigm for TRH?
RDN is increasingly significant, validated by recent robust clinical trial data showing sustained, non-pharmacological blood pressure reduction. It is anticipated to become a cornerstone therapy, particularly for patients with poor adherence to medication or those who have significant sympathetic nervous system overactivity, offering a durable interventional alternative.
Which drug classes are most effective and showing the fastest growth in the TRH pharmacological segment?
Mineralocorticoid Receptor Antagonists (MRAs), such as spironolactone, are highly effective and demonstrate rapid growth because primary aldosteronism is a common underlying mechanism in TRH. Additionally, novel non-steroidal MRAs and specialized vasodilators are emerging as key growth drivers due to improved side-effect profiles.
Which geographical region leads the market for advanced TRH management technologies?
North America, specifically the United States, leads the market due to its advanced cardiology infrastructure, high willingness to adopt innovative medical devices like RDN, high procedural volumes, and favorable, though complex, reimbursement structures for specialized cardiovascular interventions.
What role does Artificial Intelligence (AI) play in the diagnosis and management of resistant hypertension?
AI is crucial for enhancing diagnostic accuracy by analyzing complex real-time blood pressure data to distinguish true TRH from pseudo-resistance. Furthermore, AI algorithms are being developed to personalize treatment by predicting the optimal drug combination or suitability for device therapies like RDN, improving efficiency and therapeutic outcomes.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
