Zika Virus Infection Drug Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 437555 | Date : Dec, 2025 | Pages : 258 | Region : Global | Publisher : MRU
Zika Virus Infection Drug Market Size
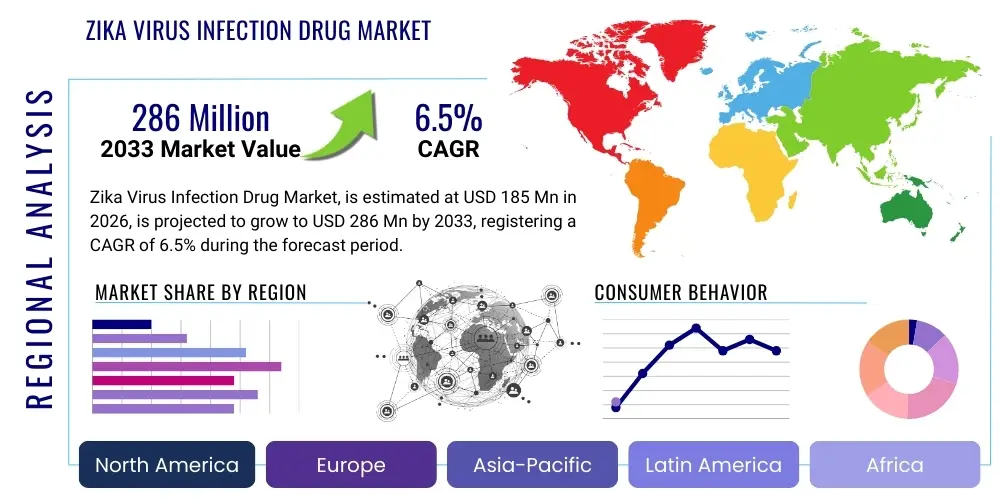
The Zika Virus Infection Drug Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 6.5% between 2026 and 2033. The market is estimated at USD 185 Million in 2026 and is projected to reach USD 286 Million by the end of the forecast period in 2033.
Zika Virus Infection Drug Market introduction
The Zika Virus Infection Drug Market encompasses the research, development, and commercialization of therapeutic agents designed to prevent, treat, or manage infection caused by the Zika virus (ZIKV), a mosquito-borne flavivirus primarily transmitted by Aedes mosquitoes. While ZIKV infection is often asymptomatic or mild in adults, its association with severe neurological complications, particularly microcephaly and other congenital anomalies collectively known as Congenital Zika Syndrome (CZS) when infection occurs during pregnancy, drives the critical need for effective pharmacological interventions. The primary focus of drug development revolves around direct-acting antivirals that target viral replication mechanisms, alongside supportive care drugs aimed at managing symptoms and related neurological sequelae.
Major applications for Zika virus drugs include proactive treatment for confirmed symptomatic cases, therapeutic management of pregnant women exposed to the virus to mitigate fetal risk, and potentially, prophylactic use in high-risk endemic or epidemic regions. Currently, the market is characterized by a strong emphasis on investigational products, as there are no FDA-approved specific antiviral treatments for ZIKV infection. Existing treatment protocols primarily involve supportive care, hydration, and the use of common pain and fever relievers. The urgency created by potential outbreaks and the devastating impact of CZS acts as a significant catalyst for investment in novel therapeutic platforms, including repurposing existing broad-spectrum antivirals and developing new small molecule inhibitors.
Key driving factors accelerating market growth include increasing global travel to endemic areas, which heightens transmission risks; climate change leading to expanded geographical reach of the Aedes mosquito vector; substantial funding from governmental and philanthropic organizations for neglected tropical diseases research; and advancements in drug discovery techniques, such as structure-based drug design and high-throughput screening. Furthermore, heightened public health awareness and improved diagnostic capabilities contribute to the identification of cases, thereby increasing the potential target population for future drug therapies. The inherent risk of future epidemics ensures sustained R&D focus from both biotech startups and established pharmaceutical giants.
Zika Virus Infection Drug Market Executive Summary
The Zika Virus Infection Drug Market is poised for moderate but impactful growth, driven primarily by the ongoing threat of ZIKV outbreaks, intense public health focus on preventing congenital anomalies, and rapid progress in preclinical and clinical testing of novel antiviral candidates. Business trends indicate a shift toward public-private partnerships, leveraging governmental and academic resources to de-risk costly and complex tropical disease drug development. Pharmaceutical companies are strategically focusing on broad-spectrum antivirals that might also be effective against other flaviviruses (like Dengue or West Nile), offering diversified revenue streams. Investment in mRNA and other innovative platform technologies for rapid response drug candidates is also a notable trend, emphasizing speed and scalability in preparation for potential future pandemics. Despite the current lack of approved drugs, the pipeline strength signals significant future market entry and disruption.
Regionally, the market is profoundly influenced by the epidemiology of ZIKV. Latin America, particularly Brazil, and parts of Southeast Asia and Africa, represent core areas of demand and clinical trial activity due to high historical prevalence and ongoing endemic risk. North America and Europe, while having lower endemic risk, serve as crucial R&D and funding hubs, driving the intellectual property and commercialization efforts. Furthermore, global health organizations' procurement strategies heavily influence market dynamics in lower- and middle-income countries, emphasizing affordable and accessible therapeutic solutions. Regulatory streamlining, particularly for drugs targeting infectious disease emergencies, is becoming a central theme in key regulatory bodies like the FDA and EMA, which impacts market entry timelines across all geographic segments.
Segmentation trends reveal a concentration of research efforts within the Antiviral Drug Type segment, specifically targeting viral entry and replication mechanisms (NS3 protease and NS5 polymerase inhibitors). In terms of application, preventing or treating Congenital Zika Syndrome (CZS) remains the most critical and valuable segment due to the high socio-economic burden associated with the condition, outweighing the market size of treating acute, typically self-limiting adult infections. The distribution landscape is expected to be dominated initially by hospital pharmacies and centralized governmental stockpiles, emphasizing rapid response and controlled distribution during outbreaks, with retail and online channels playing a more supportive role for symptomatic relief and post-outbreak prophylaxis.
AI Impact Analysis on Zika Virus Infection Drug Market
Users frequently inquire about how artificial intelligence (AI) and machine learning (ML) are accelerating the identification of promising drug candidates, reducing R&D timelines, and improving the success rate of clinical trials in infectious disease domains like Zika. Key themes revolve around AI's ability to analyze vast genomic and proteomic datasets of the Zika virus, predict optimal drug targets, screen vast libraries of existing molecules for repurposing potential, and design novel small molecules or biologics with high specificity. Concerns often center on data privacy in patient monitoring using AI tools, the validation process for AI-identified targets, and the ethical implications of using predictive analytics for outbreak forecasting and resource allocation. Expectations are high regarding AI's role in making drug development for neglected tropical diseases more cost-effective and rapid, mitigating the financial risks often associated with these markets.
- AI-driven identification of novel ZIKV enzyme inhibitors (e.g., NS3 and NS5) using deep learning models analyzing protein structures.
- Accelerated virtual screening of large compound libraries, significantly reducing the timeline for hit-to-lead optimization in drug discovery.
- Machine learning algorithms predicting drug efficacy and toxicity profiles early in the preclinical phase, filtering out less promising candidates.
- Enhanced predictive modeling for ZIKV outbreak forecasting and geographic spread, aiding in the strategic deployment of clinical trials and drug stockpiles.
- Optimization of clinical trial design and patient recruitment strategies using AI to analyze demographic and viral load data, improving trial efficiency for low-prevalence diseases.
- Application of Natural Language Processing (NLP) to synthesize global research literature, identifying potential drug synergies or overlooked targets specific to ZIKV neurotropism.
DRO & Impact Forces Of Zika Virus Infection Drug Market
The market dynamics for Zika Virus Infection Drugs are shaped by a complex interplay of public health emergencies, scientific advancements, and funding availability. Drivers include the recurring epidemic threat and the devastating long-term costs of Congenital Zika Syndrome (CZS), which pressure governments and NGOs to invest proactively in therapeutic solutions. Restraints largely stem from the unpredictable and cyclical nature of ZIKV outbreaks, leading to 'boom and bust' cycles in funding and R&D focus, alongside the inherent difficulty in designing clinical trials for diseases with sporadic prevalence. Opportunities lie in developing broad-spectrum antiviral agents effective against the entire Flavivirus family and leveraging advanced platform technologies like mRNA for rapid therapeutic development. The overall impact forces center on geopolitical commitment to global health security and the successful translation of preclinical discoveries into affordable, accessible commercial products, particularly for vulnerable populations in endemic regions.
Specific drivers include robust governmental and international funding mechanisms, such as those provided by CEPI or WHO, dedicated to accelerating R&D for outbreak-prone diseases. The advancement of molecular biology techniques allows for precise targeting of viral mechanisms, increasing the likelihood of developing potent antivirals. Furthermore, the established regulatory pathway flexibility (e.g., Fast Track designation) for infectious disease threats incentivizes pharmaceutical companies. However, significant restraints impede faster progress. The relatively short duration of acute ZIKV infection often makes definitive clinical endpoint trials challenging, and the ethical complexity surrounding trials involving pregnant women requires stringent oversight, slowing down Phase II and III testing. The high cost of R&D for a disease primarily affecting developing nations also presents a commercialization hurdle, demanding creative pricing and procurement models.
Market opportunities are expansive within the pediatric and maternal health segment, specifically focusing on therapeutics that can safely cross the placental barrier or blood-brain barrier to treat fetal infection or neuroinflammation. Developing combination therapies, similar to HIV treatment paradigms, could mitigate resistance development and enhance efficacy. Impact forces demanding attention include the rising incidence of co-infection with other mosquito-borne illnesses (e.g., Dengue), requiring drugs compatible with existing treatment regimens, and the imperative for equitable access, influencing pricing negotiations and intellectual property management strategies. Ultimately, sustained political will to prevent future public health crises remains the strongest impact force dictating long-term market viability.
Segmentation Analysis
The Zika Virus Infection Drug Market is analyzed based on critical dimensions including Drug Type, Route of Administration, Application, and Distribution Channel, reflecting both the current pipeline landscape and anticipated commercial structure. Drug Type segmentation differentiates between specific ZIKV-targeting antivirals (currently investigational) and generic supportive care drugs used for symptom management. Application analysis is crucial, prioritizing the high-value segment of Congenital Zika Syndrome (CZS) prevention/treatment over standard acute infection treatment. These segmentations help stakeholders understand where R&D investment is most concentrated and where future revenue streams are likely to originate, guiding resource allocation in a highly specialized therapeutic area.
The segmentation by Route of Administration primarily covers oral formulations, which offer patient convenience for long-term or prophylactic use, and injectable options, often favored in severe hospitalized cases or for certain complex biological treatments like monoclonal antibodies (mAbs). The Distribution Channel segment illustrates the pathway from manufacturer to patient, with centralized procurement by governments and NGOs being paramount during large-scale public health responses, alongside standard commercial pharmacy channels for non-emergency or symptomatic products. The intersection of these segments highlights the need for a robust supply chain capable of scaling rapidly during epidemic periods while maintaining a steady supply of essential symptomatic relief agents in endemic zones.
- By Drug Type
- Specific Antiviral Agents (Investigational)
- NS3 Protease Inhibitors
- NS5 Polymerase Inhibitors
- Entry Inhibitors
- Broad-Spectrum Antivirals (Repurposed Drugs)
- Symptomatic Treatment Drugs
- NSAIDs (Non-Steroidal Anti-Inflammatory Drugs)
- Acetaminophen (Paracetamol)
- IV Fluids and Electrolytes
- Specific Antiviral Agents (Investigational)
- By Application
- Congenital Zika Syndrome (CZS) Treatment and Prevention
- Acute Zika Infection Treatment (Adults and Non-Pregnant Individuals)
- By Route of Administration
- Oral
- Injectable (Intravenous, Subcutaneous)
- Topical/Others
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Governmental Stockpiles and Public Health Agencies
- Online Pharmacies
Value Chain Analysis For Zika Virus Infection Drug Market
The value chain for the Zika Virus Infection Drug Market begins with upstream activities heavily focused on high-level academic and biotechnological research, specifically target identification (genomics, proteomics) and early-stage compound screening. Given the public health nature of the disease, initial R&D is often funded by government grants or non-profit organizations, mitigating the inherent financial risk for private biopharma companies. Upstream segments are characterized by strong intellectual property creation, demanding specialized expertise in virology and drug chemistry. Manufacturing is a critical midstream step, requiring high-containment facilities and the capability to scale production rapidly, especially for complex biologicals or novel small molecules, often through Contract Manufacturing Organizations (CMOs).
Downstream activities involve extensive clinical trials, which must navigate specific challenges related to pregnant women and children. This stage necessitates deep collaboration with public health entities in endemic regions. The distribution channel is bifurcated: direct government-to-government sales or centralized procurement by global health funds (like WHO or PAHO) dominate the delivery of highly specific antiviral agents during epidemics. This direct channel ensures rapid and controlled allocation. Conversely, symptomatic treatments often flow through indirect channels utilizing established wholesale distributors and local retail pharmacy networks.
The value capture largely occurs at the R&D and manufacturing stages. Direct distribution, although low-margin in some government tenders, provides significant market volume stability during public health crises. Key strategic considerations involve securing intellectual property rights early and establishing robust collaborations with regional diagnostic providers, as timely and accurate diagnosis directly impacts drug utilization. Efficiency in the value chain is crucial, given the sporadic demand, emphasizing preparedness and rapid deployment capabilities across all segments.
Zika Virus Infection Drug Market Potential Customers
The primary customers in the Zika Virus Infection Drug Market are governmental bodies, public health institutions, and large non-governmental organizations responsible for disease control and emergency preparedness. These institutional buyers, including Ministries of Health, the World Health Organization (WHO), and regional public health agencies, purchase drugs in large volumes for national stockpiles and to deploy during outbreaks, serving the collective population. Their purchasing decisions are heavily influenced by efficacy data, WHO prequalification status, and unit cost affordability, especially in low- and middle-income countries (LMICs).
A secondary, yet crucial, customer segment includes healthcare providers in endemic regions, such as major hospitals, infectious disease clinics, and maternal health centers. These institutions purchase and administer the drugs directly to confirmed or suspected patients, particularly pregnant women identified as high-risk. Furthermore, specific research institutions and clinical trial sponsors represent immediate customers for specialized, early-stage drug formulations needed for ongoing investigation. The end-users, the patients (especially pregnant women and newborns with CZS), benefit from the treatment, though they are not the direct purchasers in the public health model.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 185 Million |
| Market Forecast in 2033 | USD 286 Million |
| Growth Rate | 6.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Gilead Sciences, Merck & Co., F. Hoffmann-La Roche, Pfizer, GlaxoSmithKline (GSK), Johnson & Johnson, Takeda Pharmaceutical, Sanofi, Moderna, BioNTech, Regeneron Pharmaceuticals, Novavax, Bharat Biotech, Bavarian Nordic, Emergent BioSolutions, ViiV Healthcare, Daiichi Sankyo, Shionogi & Co., Genentech, Astellas Pharma |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Zika Virus Infection Drug Market Key Technology Landscape
The technology landscape driving the Zika Virus Infection Drug Market is characterized by innovation across molecular target identification and therapeutic modality development. Key technologies include advanced high-throughput screening (HTS) techniques, which enable the rapid testing of tens of thousands of chemical compounds against ZIKV enzymes (like NS3 protease or NS5 polymerase) to identify potential inhibitors. Structure-based drug design (SBDD) is crucial, allowing researchers to visualize the viral protein structures and computationally design small molecules that fit precisely into the active sites, thereby improving potency and reducing off-target effects. This approach drastically shortens the lead optimization phase, which is vital for quick epidemic response.
Furthermore, platforms for developing biological therapeutics, particularly monoclonal antibodies (mAbs), represent a significant technological focus. Neutralizing mAbs capable of blocking viral entry into host cells, including placental and neuronal cells, are being explored for passive immunization or treatment of active infection, especially in pregnant patients. The technology relies on advanced hybridoma techniques, single B-cell sorting, and large-scale recombinant protein manufacturing. These complex biologics often require specialized delivery systems but offer high specificity and potentially rapid protective effects, making them highly valuable during public health emergencies.
Finally, the integration of genomic and transcriptomic technologies plays a crucial role in understanding viral pathogenesis and host response. This includes Next-Generation Sequencing (NGS) to monitor viral mutation and evolution, and CRISPR-Cas systems, which are being investigated both as direct antiviral agents to cleave the viral genome and as research tools to validate novel host factors essential for ZIKV replication. The convergence of computational biology, advanced molecular screening, and innovative delivery platforms defines the cutting-edge of therapeutic development in this specific infectious disease market.
Regional Highlights
- Latin America (LATAM): LATAM remains the epicenter of ZIKV activity and, consequently, the primary end-user market. Countries like Brazil, Colombia, and Mexico have borne the highest burden of historical cases and CZS incidence, making them critical for late-stage clinical trials and governmental procurement of future approved therapies. Market dynamics here are strongly influenced by public healthcare budgets, international aid, and the necessity for highly affordable drug pricing. Demand is focused on preventative measures for pregnant women and treating the neurological sequelae of CZS.
- Asia Pacific (APAC): The APAC region, especially Southeast Asia (e.g., Thailand, Vietnam, Philippines), faces endemic ZIKV transmission alongside other co-circulating flaviviruses like Dengue. This region presents a massive potential market due to high population density and ecological suitability for the Aedes mosquito vector. Market growth in APAC is driven by increasing investment in regional biotechnology centers and the development of local manufacturing capabilities for rapid deployment across diverse healthcare systems. Strategic preparedness is a major governmental priority, fueling interest in stockpiling broad-spectrum antivirals.
- North America (NA): While largely non-endemic, North America, particularly the United States, dominates the market in terms of R&D investment, technological innovation, and regulatory approvals (FDA). Major pharmaceutical and biotech companies leading the Zika drug pipeline are headquartered here. The market is defined by high academic research output and substantial governmental funding through agencies like NIH and BARDA, aiming to protect against importation and potential localized transmission risks in southern states. Pricing here, for any future approved drugs, is expected to be premium, supporting R&D returns.
- Europe: Europe serves as a significant hub for pharmaceutical R&D, clinical trial sponsorship, and regulatory oversight (EMA). European countries focus on managing travel-related ZIKV cases and supporting international efforts through organizations like the European Commission and various national public health institutes. The market contribution is primarily through funding, technological input, and the distribution of supportive care products, with limited immediate consumer demand, emphasizing preparedness and global health contributions.
- Middle East and Africa (MEA): Africa represents a long-standing but often under-monitored endemic region for ZIKV. Market development in MEA is challenged by fragmented healthcare infrastructure and limited financial resources, but the necessity for effective drugs is immense. Demand here is almost entirely dependent on international aid and global philanthropic initiatives. The Middle East, due to high international travel volumes and potential for local transmission, invests in robust surveillance and preparedness systems, focusing on diagnostic advancements alongside therapeutic development.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Zika Virus Infection Drug Market.- Gilead Sciences
- Merck & Co.
- F. Hoffmann-La Roche
- Pfizer
- GlaxoSmithKline (GSK)
- Johnson & Johnson
- Takeda Pharmaceutical
- Sanofi
- Moderna
- BioNTech
- Regeneron Pharmaceuticals
- Novavax
- Bharat Biotech
- Bavarian Nordic
- Emergent BioSolutions
- ViiV Healthcare
- Daiichi Sankyo
- Shionogi & Co.
- Genentech
- Astellas Pharma
Frequently Asked Questions
Analyze common user questions about the Zika Virus Infection Drug market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the demand for Zika Virus drugs?
The primary driver is the critical need to prevent Congenital Zika Syndrome (CZS), which causes severe neurological birth defects (microcephaly), coupled with the recurring threat of large-scale epidemics in endemic and vulnerable non-endemic regions.
Are there currently any FDA-approved specific antiviral treatments for Zika Virus infection?
No, there are currently no specific antiviral drugs approved by the FDA or EMA solely for treating Zika Virus infection. Treatment relies on supportive care (like pain relievers) while numerous specific antiviral candidates are in preclinical or clinical development.
How does AI contribute to Zika drug development efforts?
AI accelerates drug discovery by performing high-throughput virtual screening of chemical libraries, predicting optimal viral protein targets (e.g., NS3/NS5), and optimizing preclinical efficacy and toxicity assessments, thereby reducing the time required to advance promising candidates.
Which geographic region holds the largest potential market share for Zika drugs?
Latin America, due to high historical prevalence and ongoing endemic risk, represents the largest potential market for governmental procurement and deployment of approved therapies, followed closely by the Asia Pacific region which faces substantial co-circulating flavivirus threats.
What are the main obstacles facing the commercialization of Zika Virus drugs?
Key obstacles include the unpredictable, cyclical nature of outbreaks (leading to fluctuating R&D focus), challenges in conducting clinical trials in pregnant women, and the requirement for low-cost solutions necessary for widespread adoption in resource-limited endemic countries.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager