Levothyroxine Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432730 | Date : Dec, 2025 | Pages : 242 | Region : Global | Publisher : MRU
Levothyroxine Market Size
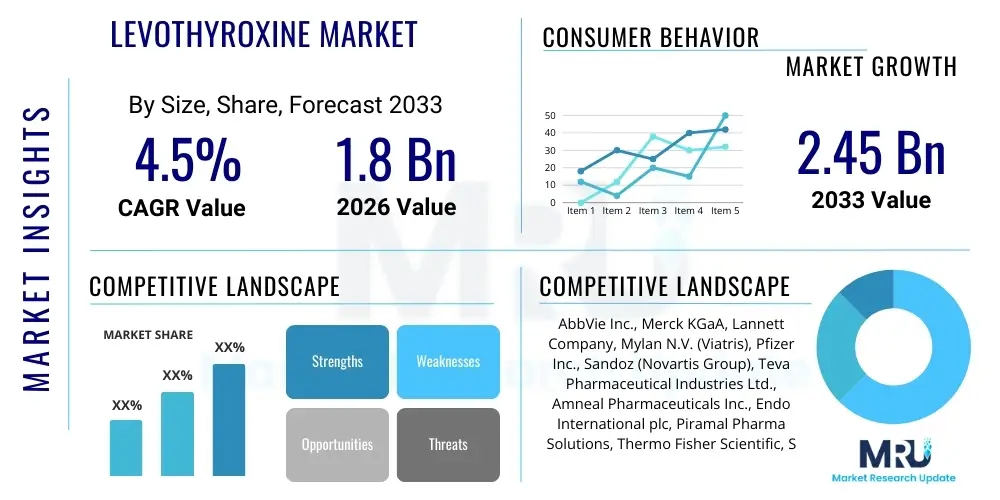
The Levothyroxine Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 4.5% between 2026 and 2033. The market is estimated at USD 1.8 Billion in 2026 and is projected to reach USD 2.45 Billion by the end of the forecast period in 2033.
Levothyroxine Market introduction
The Levothyroxine Market encompasses the global sales and distribution of synthetic T4 hormone, primarily used for the treatment of hypothyroidism, a prevalent endocrine disorder characterized by inadequate production of thyroid hormones. Levothyroxine sodium is the standard of care due to its long half-life, reliable absorption, and established efficacy in restoring normal thyroid hormone levels, thereby alleviating symptoms such as fatigue, weight gain, and depression. The increasing global prevalence of thyroid disorders, coupled with enhanced screening programs and improved diagnostic accuracy, is fundamentally driving market expansion across all major economies.
Levothyroxine is available in various formulations, predominantly tablets, but also increasingly in liquid and gel capsule forms designed to optimize bioavailability and address patient compliance issues, particularly in sensitive populations such as neonates or patients with complex absorption challenges. The major applications of this synthetic hormone extend beyond primary hypothyroidism to include secondary and tertiary hypothyroidism, congenital hypothyroidism management, and as a thyroid-stimulating hormone (TSH) suppression agent in patients treated for thyroid cancer. The sustained demand is underpinned by the chronic nature of hypothyroidism, requiring lifelong medication management, which ensures a steady and predictable revenue stream for manufacturers.
Key driving factors for market growth include the aging population, which is inherently more susceptible to thyroid dysfunction; greater awareness leading to higher rates of diagnosis; and technological advancements focused on developing bioequivalent and stable formulations. Furthermore, government initiatives in developed nations aimed at controlling chronic diseases and providing subsidized access to essential medicines contribute significantly to the market's stability. However, the market faces constraints related to intense generic competition and the necessity for precise dosage titration, which complicates the standardization of treatment protocols across diverse patient demographics.
Levothyroxine Market Executive Summary
The Levothyroxine Market is characterized by mature product lines dominated by generics, yet experiencing significant growth driven by epidemiological factors and advancements in drug delivery systems. Business trends indicate a strong focus on enhancing formulation stability and shelf-life, particularly in sensitive dosage forms like liquids, to maintain brand differentiation against low-cost generic alternatives. Leading pharmaceutical companies are strategically investing in emerging markets, especially in Asia Pacific, where rising incomes and improved healthcare infrastructure are facilitating better access to diagnosis and treatment for previously undiagnosed thyroid conditions. Moreover, competitive dynamics are centered on demonstrating bioequivalence and superior patient outcomes, with a notable shift towards specialized compounding pharmacies catering to customized dosing needs.
Regional trends highlight North America as the dominant market, primarily due to high healthcare expenditure, established screening protocols, and a large patient base using premium branded or authorized generic products. Europe follows closely, driven by sophisticated regulatory frameworks and comprehensive public healthcare coverage ensuring wide treatment availability. Conversely, the Asia Pacific region is projected to register the highest Compound Annual Growth Rate (CAGR), fueled by expanding middle-class populations, increasing prevalence of lifestyle-related thyroid disorders, and significant governmental investment in chronic disease management programs. The market structure varies regionally, with highly regulated, fixed-price environments in some European countries contrasting with more price-competitive, open markets in North America and parts of Asia.
Segment trends reveal that the tablet formulation remains the cornerstone of the market due to its cost-effectiveness and ease of use, though the liquid and soft gel capsule segments are gaining momentum, particularly for patients exhibiting poor absorption or compliance issues with traditional tablets. The application segment remains dominated by primary hypothyroidism, which accounts for the vast majority of prescriptions globally. Distribution channel analysis shows that retail pharmacies maintain the largest share, serving the needs of chronic care patients; however, online pharmacies are rapidly expanding their footprint, capitalizing on convenience, competitive pricing, and the recurring nature of Levothyroxine prescriptions, which align perfectly with e-commerce models.
AI Impact Analysis on Levothyroxine Market
Common user questions regarding AI's impact on the Levothyroxine market revolve around precision dosing, diagnostic efficiency, and accelerating drug development for novel thyroid treatments. Users frequently inquire if Artificial Intelligence can personalize Levothyroxine dosage titration better than current empirical methods, given the hormone's narrow therapeutic index and high patient variability. There is significant interest in how machine learning algorithms can analyze vast amounts of patient data, including genomic, lifestyle, and co-morbidity factors, to predict optimal initial and maintenance doses, minimizing the time required to achieve euthyroid status. Furthermore, stakeholders seek clarification on AI's role in accelerating the discovery of superior thyroid hormone alternatives or delivery mechanisms that overcome existing formulation stability and absorption challenges inherent in Levothyroxine sodium.
In the diagnostic realm, AI is already transforming how thyroid dysfunction is identified. Machine learning algorithms are being integrated with imaging technologies, such as ultrasound and thyroid scintigraphy, to improve the accuracy of nodule detection and risk stratification, potentially reducing unnecessary biopsies and accelerating the initiation of Levothyroxine therapy where appropriate. These systems analyze patterns invisible to the human eye, predicting malignancy likelihood or identifying subtle physiological indicators of impending hypothyroidism, thereby shifting clinical practice towards proactive intervention. This enhancement in diagnostic precision translates directly into earlier and more effective market penetration for Levothyroxine products, driven by evidence-based medicine facilitated by AI insights.
Manufacturing and supply chain logistics for Levothyroxine, a high-volume, commodity drug, also stand to benefit significantly from AI optimization. Predictive maintenance models powered by AI ensure pharmaceutical production lines maintain high uptime and product quality, crucial for maintaining the large, consistent supply required globally. Furthermore, AI-driven supply chain forecasting helps manufacturers manage inventory levels more accurately, anticipating regional demand fluctuations and mitigating risks of stockouts, which are particularly critical for essential, chronic medications like Levothyroxine. The optimization of formulation stability testing and quality control through computer vision and predictive modeling represents a crucial operational efficiency gain enabled by AI integration.
- Enhanced Personalized Dosing: AI algorithms analyze patient biomarkers and clinical response data to suggest precise Levothyroxine dosage adjustments, minimizing the risk of over or under-treatment.
- Accelerated Drug Discovery: Machine learning is utilized to screen potential compounds for improved stability or novel T4 analogs, reducing R&D timelines for next-generation treatments.
- Improved Diagnostic Accuracy: Integration of AI with imaging and lab results for faster, more accurate detection and staging of thyroid disorders requiring Levothyroxine treatment.
- Optimized Manufacturing Quality: AI-powered predictive maintenance and quality control systems ensure consistent high standards and reliable production capacity for high-volume manufacturing.
- Supply Chain Efficiency: Predictive analytics forecasts demand shifts, optimizing inventory management and distribution networks to prevent shortages of essential medication.
DRO & Impact Forces Of Levothyroxine Market
The Levothyroxine Market is propelled by significant demographic drivers, notably the increasing prevalence of thyroid disorders, particularly hypothyroidism, driven by aging populations and greater awareness, which necessitates long-term, chronic management using synthetic T4 hormone. Simultaneously, the market is constrained by the widespread availability of low-cost generic versions and bioequivalence challenges that often lead to regulatory scrutiny and complicate the substitution of branded products. Opportunities for growth lie primarily in developing advanced delivery systems, such as liquid or sustained-release formulations, designed to improve absorption, stability, and patient compliance, especially within challenging patient populations or in high-humidity environments. These combined forces shape the market trajectory, where demand remains inelastic but pricing and competitive advantages are highly sensitive to formulation innovation and operational efficiency.
Drivers influencing the market include public health campaigns promoting thyroid health, especially those targeting pregnant women and newborns, which contribute to early diagnosis and lifelong treatment initiation. Furthermore, advancements in diagnostic technologies, including easier access to TSH testing in primary care settings, expand the pool of diagnosed patients requiring therapy. However, the primary restraint is the regulatory hurdle associated with demonstrating true bioequivalence for generic Levothyroxine products; small differences in dissolution rates can significantly impact clinical effectiveness due to the drug's narrow therapeutic index, prompting caution among prescribers and potentially maintaining a segment loyal to established, branded products despite cost differences.
The impact forces are substantial, creating both market friction and innovation pressure. The opportunity landscape is rich in the area of formulation science, focusing on solving the inherent stability issues of Levothyroxine sodium, which is sensitive to moisture, light, and excipients. Developing formulations that maintain potency across diverse climatic zones represents a critical competitive advantage, especially when targeting rapidly growing emerging markets. Furthermore, the push towards personalized medicine opens avenues for customized dosing solutions and combination therapies, such as Levothyroxine and Liothyronine (T3) combinations, offering potential therapeutic benefits for patients who do not fully respond to T4 monotherapy. These innovations mitigate the restrictive forces of generic substitution by providing clinically meaningful differentiation.
Segmentation Analysis
The Levothyroxine market segmentation provides a granular view of market dynamics based on formulation type, clinical application, and distribution channel. Formulation segmentation is critical, highlighting the traditional tablet dominance while tracking the rapid adoption of alternative liquid and capsule forms offering improved bioavailability and stability. Application analysis underscores the vast majority of market demand originating from primary hypothyroidism, defining the core demographic and treatment protocols. Distribution channel segmentation reflects the mature nature of the chronic medication market, heavily reliant on established pharmacy networks, increasingly supplemented by convenient online dispensing models.
- By Formulation
- Tablet
- Capsule (Soft Gel)
- Liquid Solution
- By Application
- Primary Hypothyroidism
- Secondary Hypothyroidism
- Congenital Hypothyroidism
- Thyroid Cancer (TSH Suppression)
- By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Value Chain Analysis For Levothyroxine Market
The Levothyroxine value chain begins with complex upstream activities focused on sourcing high-purity raw materials and the intricate synthesis of Levothyroxine sodium (L-thyroxine). This critical phase demands stringent quality control, as the purity and stability of the Active Pharmaceutical Ingredient (API) directly impact the final drug product's efficacy and bioequivalence. Manufacturers must adhere to rigorous Good Manufacturing Practice (GMP) standards due to the potent nature and narrow therapeutic index of the hormone. Efficient upstream management involves securing stable, long-term contracts for precursors and optimizing chemical synthesis yields to manage production costs in a highly price-sensitive generic environment.
Midstream activities encompass formulation, manufacturing, and packaging. Levothyroxine requires specialized formulation techniques to ensure dosage uniformity and stability against environmental factors such as moisture and heat. Companies invest heavily in advanced tableting and encapsulation technologies, particularly for developing new, stable liquid or soft gel formulations that offer better patient compliance and absorption. Direct distribution involves manufacturers supplying large volumes directly to institutional buyers like hospitals and major pharmacy chains. Indirect distribution, which accounts for the largest volume, utilizes wholesalers and distributors who manage regional inventory, logistics, and delivery to independent retail pharmacies, ensuring broad market access for chronic medication prescriptions.
Downstream activities center on distribution channels, including both direct patient dispensing and indirect sales through managed care organizations. Retail pharmacies constitute the primary point of sale, managing patient refill cycles and often providing patient education. Online pharmacies are rapidly gaining importance by offering mail-order convenience, especially valuable for chronic, routine medications like Levothyroxine, driving efficiencies in the downstream segment. The market heavily relies on established physician prescription patterns, with promotional efforts often directed towards demonstrating superior bioequivalence data or addressing specific formulation needs (e.g., allergen-free or dye-free options) to capture market share at the point of prescription.
Levothyroxine Market Potential Customers
The primary end-users and buyers of Levothyroxine are chronic care patients diagnosed with hypothyroidism, representing a large, stable, and continuously growing customer base that requires lifelong medication management. This demographic spans all age groups, though incidence rates are significantly higher among women and individuals over the age of 60. Healthcare providers, including endocrinologists, primary care physicians, and pediatricians, act as the crucial decision-makers (prescribers) who guide the purchasing choices of patients, heavily favoring products with proven consistency, bioequivalence, and reliable supply chains. Institutional buyers, such as hospitals and large health management organizations (HMOs), represent key purchasing entities focused on volume discounts and inclusion in formulary lists.
A significant segment of potential customers includes specialized populations that present unique formulation challenges, such as infants with congenital hypothyroidism who require liquid formulations for precise micro-dosing, or patients undergoing bariatric surgery who may experience altered drug absorption, necessitating fast-acting or liquid alternatives. Furthermore, individuals being treated for specific types of thyroid cancer are high-volume users, requiring TSH suppression regimens often involving higher dosages of Levothyroxine. Manufacturers target these niche populations by developing specialized, stable dosage forms, aiming to secure premium pricing and brand loyalty where generic competition is less aggressive due to formulation complexity.
Managed care organizations, government health programs (like Medicare/Medicaid in the US or national health services globally), and private insurance companies serve as major financial customers, as they determine reimbursement policies and formulary coverage, directly impacting which products are commercially viable and accessible to the patient population. These institutional customers prioritize cost-effectiveness and proven therapeutic outcomes. Consequently, market success hinges not only on product quality but also on demonstrating favorable pharmacoeconomic data and securing favorable formulary placement through strategic negotiation and volume purchasing agreements with pharmaceutical suppliers.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 1.8 Billion |
| Market Forecast in 2033 | USD 2.45 Billion |
| Growth Rate | 4.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | AbbVie Inc., Merck KGaA, Lannett Company, Mylan N.V. (Viatris), Pfizer Inc., Sandoz (Novartis Group), Teva Pharmaceutical Industries Ltd., Amneal Pharmaceuticals Inc., Endo International plc, Piramal Pharma Solutions, Thermo Fisher Scientific, Sanofi S.A., Bristol-Myers Squibb, Bayer AG, Cipla Ltd., Sun Pharmaceutical Industries Ltd., Dr. Reddy's Laboratories, GlaxoSmithKline (GSK), Aurobindo Pharma Limited, Daiichi Sankyo Company, Limited. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Levothyroxine Market Key Technology Landscape
The core technology surrounding the Levothyroxine market centers on formulation science aimed at enhancing the chemical stability and bioavailability of the API. Levothyroxine sodium is highly prone to degradation when exposed to humidity, light, or specific excipients like corn starch or certain food components, which can severely impact its potency and, consequently, patient outcomes. Key technological advancements focus on developing anhydrous or highly stabilized formulations, such as those using gelatin capsules or specialized liquid carriers, to shield the API from environmental degradation and ensure consistent drug delivery throughout its shelf life. Microencapsulation and advanced coating techniques are also employed to control dissolution rates and maintain therapeutic equivalence across batches, addressing a fundamental challenge in generic Levothyroxine manufacturing.
Another crucial area in the technology landscape involves advanced analytics and process automation in manufacturing. Given the extremely low dosage levels and the need for high batch consistency (often measured in micrograms), sophisticated blending and quality control technologies are essential. High-shear granulation and precision mixing equipment ensure uniform distribution of the API within the excipient matrix. Furthermore, spectroscopic and chromatographic techniques, including High-Performance Liquid Chromatography (HPLC) coupled with mass spectrometry, are utilized extensively for rigorous analytical testing, guaranteeing both product purity and accurate quantification of the active ingredient, critical for regulatory compliance and safety.
The emerging technological focus is on delivery systems designed to improve patient adherence and absorption. This includes the development of stable liquid Levothyroxine formulations that bypass absorption interference issues common with food or certain medications when administered in tablet form. These liquid forms often utilize specific buffer systems and preservatives to maintain stability while offering dosing flexibility, especially useful in pediatric or critically ill patients. Future technology is exploring sustained-release systems or transdermal patches, though these are currently in early-stage development, aimed at providing more stable serum T4 concentrations and potentially reducing the burden of daily medication intake for chronic patients.
Regional Highlights
- North America (Dominant Market): The U.S. and Canada represent the largest revenue generators due to high prevalence rates of hypothyroidism, established and robust diagnostic screening protocols, and high per capita healthcare spending. The market is characterized by a strong presence of both branded (like Synthroid) and highly utilized authorized generic versions. Reimbursement policies and strong regulatory scrutiny over bioequivalence maintain consumer loyalty to trusted products.
- Europe (Stable Growth): Western European countries, particularly Germany, the UK, and France, exhibit stable growth driven by universal healthcare systems that ensure wide patient access to treatment. Market dynamics are heavily influenced by national pricing and reimbursement bodies, leading to intense competition among generics, although patented specialty formulations can command higher prices.
- Asia Pacific (Highest CAGR): This region, encompassing China, India, and Japan, is expected to post the fastest growth, primarily fueled by massive population bases, rising incidence of lifestyle-related thyroid disorders, and improving diagnostic infrastructure. Rapid urbanization and increasing disposable income are translating into greater patient access to prescription pharmaceuticals.
- Latin America (Emerging Potential): Growth in countries like Brazil and Mexico is supported by expanding government health programs and increasing health awareness. The market is often fragmented, with local manufacturers competing against multinational firms, resulting in competitive pricing pressures and varied regulatory environments.
- Middle East and Africa (Niche Growth): Growth is steady, focused mainly on Gulf Cooperation Council (GCC) nations with high healthcare spending. Challenges include varied patient access and infrastructure limitations in parts of Africa, although the increasing adoption of Western treatment protocols drives demand for standardized pharmaceutical products.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Levothyroxine Market.- AbbVie Inc.
- Merck KGaA
- Lannett Company
- Mylan N.V. (Viatris)
- Pfizer Inc.
- Sandoz (Novartis Group)
- Teva Pharmaceutical Industries Ltd.
- Amneal Pharmaceuticals Inc.
- Endo International plc
- Piramal Pharma Solutions
- Thermo Fisher Scientific
- Sanofi S.A.
- Bristol-Myers Squibb
- Bayer AG
- Cipla Ltd.
- Sun Pharmaceutical Industries Ltd.
- Dr. Reddy's Laboratories
- GlaxoSmithKline (GSK)
- Aurobindo Pharma Limited
- Daiichi Sankyo Company, Limited
Frequently Asked Questions
Analyze common user questions about the Levothyroxine market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the Levothyroxine Market growth?
The increasing global prevalence of hypothyroidism, particularly among the aging population and women, combined with enhanced diagnostic screening programs, is the primary driver ensuring continuous, lifelong demand for Levothyroxine products.
Why are liquid and capsule formulations of Levothyroxine gaining popularity?
Liquid and soft gel capsule formulations are gaining traction because they often offer improved bioavailability, are less susceptible to absorption interference from food or other medications, and enhance stability against environmental degradation compared to traditional tablets.
What is the main restraint impacting the profitability of Levothyroxine manufacturers?
The primary restraint is intense price competition stemming from the high availability and regulatory approval of numerous low-cost generic substitutes, which pressure the pricing power of branded and specialized Levothyroxine products.
How does Artificial Intelligence (AI) influence Levothyroxine treatment?
AI is beginning to influence treatment by enabling personalized medicine, particularly through the use of algorithms to recommend optimal, precise dosing schedules based on patient-specific physiological data, minimizing titration time and side effects.
Which geographical region is projected to experience the highest market growth rate?
The Asia Pacific region is forecast to exhibit the highest Compound Annual Growth Rate (CAGR), driven by improving healthcare access, rising disposable incomes, and the vast, previously untapped patient populations in countries like China and India.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
