Magnesium Bisglycinate API Market Size By Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), By Statistics, Trends, Outlook and Forecast 2026 to 2033 (Financial Impact Analysis)
ID : MRU_ 432846 | Date : Dec, 2025 | Pages : 243 | Region : Global | Publisher : MRU
Magnesium Bisglycinate API Market Size
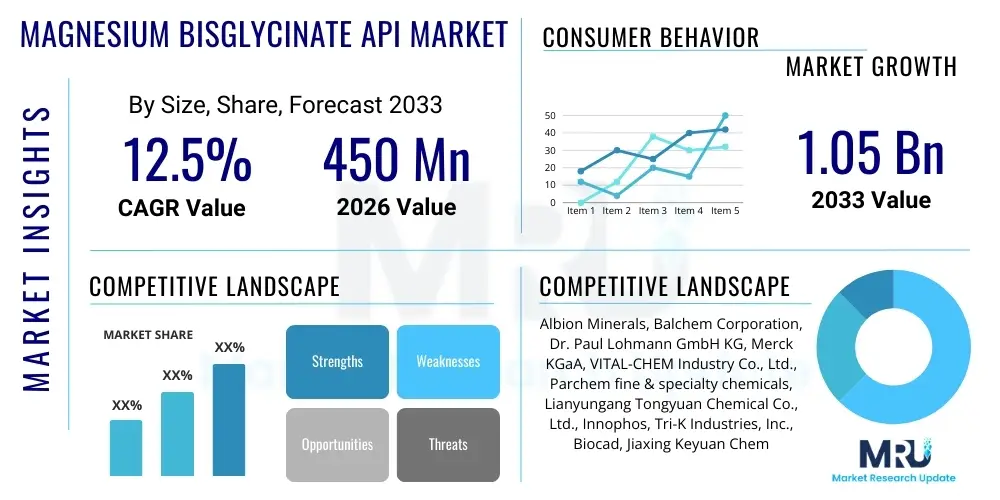
The Magnesium Bisglycinate API Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 12.5% between 2026 and 2033. The market is estimated at USD 450 Million in 2026 and is projected to reach USD 1.05 Billion by the end of the forecast period in 2033.
Magnesium Bisglycinate API Market introduction
The Magnesium Bisglycinate Active Pharmaceutical Ingredient (API) Market encompasses the production and distribution of the highly bioavailable form of magnesium chelated with two molecules of glycine. This specific chelation enhances solubility and stability, significantly improving intestinal absorption compared to conventional magnesium salts like oxide or sulfate, thereby minimizing gastrointestinal side effects. As consumers increasingly prioritize preventative healthcare and dietary supplements, the demand for high-efficacy mineral forms, particularly those targeted for cognitive health, sleep management, and muscle function recovery, has surged, positioning Magnesium Bisglycinate as a premium offering in the nutraceutical and pharmaceutical sectors.
Magnesium Bisglycinate API is primarily utilized in manufacturing dietary supplements, functional foods, and certain prescription medications where superior bioavailability is critical. Its major applications span several therapeutic areas, including cardiovascular support, management of chronic fatigue syndrome, anxiety reduction, and improvement of bone density. The inherent benefits of this form—including neutral flavor, high tolerability, and rapid uptake—make it a preferred ingredient for formulators seeking to create premium, efficacy-driven products. The versatility of the API allows for integration into capsules, tablets, powders, and liquid formulations, serving a diverse global patient and consumer base.
The driving factors behind market expansion include the global increase in magnesium deficiency awareness, particularly in developed economies, coupled with a robust shift toward clean-label supplements. Regulatory standards ensuring the purity and chelation efficiency of APIs are becoming more stringent, favoring established suppliers capable of providing certified high-grade Magnesium Bisglycinate. Furthermore, ongoing clinical research demonstrating the specific physiological advantages of bisglycinate over non-chelated forms continues to validate its premium market position, stimulating investment in advanced synthesis technologies and scalable production capacity to meet growing global demand.
Magnesium Bisglycinate API Market Executive Summary
The Magnesium Bisglycinate API market is characterized by robust growth, driven primarily by favorable consumer preferences toward highly bioavailable mineral supplements and increasing validation of its efficacy in managing lifestyle diseases such as stress and insomnia. Key business trends involve intense competition among specialized API manufacturers focusing on achieving higher purity levels and utilizing advanced, sustainable synthesis methods to reduce costs and environmental impact. Strategic alliances between API producers and large nutraceutical manufacturers are common, aimed at securing stable supply chains and leveraging specialized formulation expertise to capture market share in rapidly expanding end-user segments like personalized nutrition.
Regional trends indicate that North America and Europe currently dominate the market, owing to high levels of health awareness, sophisticated regulatory frameworks supporting dietary supplements, and significant expenditure on preventative healthcare products. However, the Asia Pacific (APAC) region is emerging as the fastest-growing market, propelled by rising disposable incomes, urbanization leading to changes in dietary habits, and the expansion of domestic pharmaceutical and supplement manufacturing capabilities in countries like China and India. This shift necessitates regional adaptation in terms of distribution and regulatory compliance strategies for global vendors.
Segment trends highlight the dominance of the nutraceutical application segment, specifically in the form of capsules and powders for general wellness and targeted mental health support. Within the purity segmentation, high-purity, pharmaceutical-grade Magnesium Bisglycinate is witnessing accelerated demand, commanding premium pricing due to its stringent quality requirements and application in clinically focused products. Furthermore, the synthesis method segment shows increasing interest in enzymatic or bio-catalytic routes, which offer pathways to higher specificity, reduced waste, and compliance with 'green chemistry' principles, positioning these advanced methods as critical differentiators for future market leaders.
AI Impact Analysis on Magnesium Bisglycinate API Market
Common user questions regarding AI's influence on the Magnesium Bisglycinate API market often revolve around how artificial intelligence can accelerate drug discovery and optimize production efficiency, particularly concerning complex chelation processes. Users frequently ask about AI's role in predicting bioavailability and formulation stability, its application in supply chain risk mitigation, and its potential impact on personalized nutrition supplement development using this specific API. The key themes emerging from this analysis center on leveraging AI for precise quality control, optimizing reaction kinetics during synthesis to ensure high yield and purity, and utilizing machine learning models to forecast fluctuating global demand based on consumer health trends and real-time market signals. Concerns primarily focus on the initial investment costs and the need for specialized data scientists to implement these sophisticated systems within traditional chemical manufacturing environments.
AI’s influence is profound, primarily focused on improving the efficiency, quality, and predictive capabilities across the API lifecycle. In the research and development phase, AI algorithms can model the optimal chelation pathways between magnesium ions and glycine molecules, predicting the structural stability and purity profile of novel synthesis methods before extensive lab work is initiated. This significantly reduces R&D cycle times and material consumption. Furthermore, machine learning models analyze large datasets of clinical trials and user feedback, identifying specific populations and physiological conditions where Magnesium Bisglycinate offers the maximum therapeutic benefit, guiding targeted product development and marketing efforts for manufacturers.
Operationally, AI systems are transforming manufacturing processes. Predictive maintenance, driven by sensor data analytics, minimizes downtime of critical reactors and purification equipment, ensuring consistent API output. More critically, AI-powered quality control systems analyze chromatographic data and spectroscopic results in real time, detecting minute impurities or deviations in chelation efficiency instantaneously, far surpassing the speed and accuracy of traditional manual inspection. This enhanced scrutiny ensures that the high-purity standards required by both pharmaceutical and premium nutraceutical segments are consistently met, bolstering supplier credibility in a highly regulated global market.
- AI-driven optimization of chemical synthesis parameters to maximize API yield and purity, minimizing resource waste.
- Predictive modeling of supply chain disruptions, allowing proactive inventory management and sourcing strategies for raw materials (magnesium salts and glycine).
- Real-time quality control using machine vision and data analytics to ensure batch consistency and adherence to strict pharmaceutical-grade specifications.
- Accelerated discovery of new excipients and delivery systems compatible with Magnesium Bisglycinate through high-throughput screening simulations.
- Personalized nutrition formulation support, utilizing AI to recommend optimal dosages and combinations based on individual patient biomarkers and genetic data.
- Enhanced demand forecasting and market trend analysis using natural language processing (NLP) of consumer health discussions and clinical literature.
DRO & Impact Forces Of Magnesium Bisglycinate API Market
The Magnesium Bisglycinate API market growth is critically influenced by a combination of strong drivers, significant restraints, and emerging opportunities, all mediated by several impactful market forces. The primary drivers include the escalating global health consciousness and the documented superior bioavailability of the bisglycinate form compared to cheaper alternatives, driving demand from premium supplement brands. However, restraints such such as the relatively high synthesis cost, complex regulatory pathways for pharmaceutical use, and price sensitivity among certain consumer segments pose challenges to widespread adoption. Opportunities are abundant in personalized medicine, utilizing the API in highly tailored formulations, and expanding into emerging economies where supplement penetration is rapidly increasing. These factors collectively dictate the market trajectory and competitive landscape for manufacturers.
Key drivers are centered around consumer demand for efficacy. The scientific evidence supporting magnesium's role in mitigating symptoms of anxiety, improving sleep quality, and supporting muscular and nervous system function directly translates into commercial success for its most effective form, Bisglycinate. Furthermore, the push towards cleaner ingredient lists and avoidance of common gastrointestinal side effects associated with cheaper inorganic magnesium salts further propels consumers and formulators toward this premium API. The increasing geriatric population worldwide, often requiring supplement support for bone density and cardiovascular health, acts as a demographic tailwind for sustained market expansion.
Conversely, the complexity of the chelation process requires specialized equipment and precise chemical controls, resulting in higher manufacturing costs compared to simpler magnesium compounds. This cost disadvantage acts as a major restraint, particularly in price-sensitive markets where generic magnesium forms dominate. Regulatory hurdles, especially in pharmaceutical applications, require exhaustive documentation and clinical validation, lengthening the time-to-market and increasing R&D expenditure for novel products utilizing the API. External impact forces, such as fluctuating raw material prices (glycine and magnesium sources) and stringent environmental regulations regarding chemical waste disposal, also influence profitability and operational scalability for API manufacturers.
Segmentation Analysis
The Magnesium Bisglycinate API market is strategically segmented based on factors crucial for differentiating product value, addressing distinct end-user needs, and optimizing production methodologies. This analysis focuses on classifications by Application (Nutraceuticals, Pharmaceuticals), Purity Level (High Purity/Pharmaceutical Grade, Standard Grade), and Synthesis Method (Chemical Synthesis, Enzymatic/Bio-catalytic Synthesis). Understanding these segments allows manufacturers to tailor their production, pricing, and marketing efforts, ensuring that the specific quality requirements of demanding segments, such as pharmaceuticals, are met while offering cost-effective solutions for high-volume nutraceutical applications. The market structure reflects the dichotomy between premium, quality-driven requirements and mass-market volume needs.
The nutraceutical segment undeniably holds the largest market share, driven by widespread consumer adoption of health supplements for general wellness, athletic performance, and mental health support. However, the pharmaceutical segment, while smaller in volume, is growing rapidly and demands the highest quality standards, pushing innovation in purification technologies. The segmentation by purity level directly correlates with application, where pharmaceutical-grade API must adhere to strict pharmacopeial standards (USP, EP), featuring negligible heavy metal contamination and consistent particle size distribution, commanding a significant price premium over standard grades typically used in lower-end dietary supplements.
Segmentation by synthesis method reveals a technological transition. Traditional chemical synthesis remains dominant due to its scalability and established processes. However, enzymatic or bio-catalytic synthesis is gaining traction. This newer method promises higher stereo-selectivity, greater process sustainability, and potentially lower long-term operating costs, aligning with industry demands for environmentally conscious manufacturing. Companies that successfully master and scale up these green chemistry techniques are expected to gain a competitive edge in serving eco-conscious brands and meeting future regulatory mandates favoring sustainable production practices.
- By Application
- Nutraceuticals (Dietary Supplements, Functional Foods, Sports Nutrition)
- Pharmaceuticals (Prescription Drugs, Over-the-counter medication)
- By Purity Level
- High Purity Grade (Pharmaceutical Grade)
- Standard Grade (Food Grade)
- By Synthesis Method
- Chemical Synthesis
- Enzymatic/Bio-catalytic Synthesis
Value Chain Analysis For Magnesium Bisglycinate API Market
The value chain for Magnesium Bisglycinate API is complex, starting from the sourcing of basic raw materials through specialized chemical processing, distribution, and final formulation into consumer products. The upstream segment involves the procurement of highly purified raw materials, primarily magnesium sources (such as magnesium chloride or magnesium oxide) and glycine. The quality and stable pricing of these foundational chemicals are crucial, as they significantly influence the final API cost and quality profile. Manufacturers often establish long-term contracts with specialized chemical suppliers to mitigate price volatility and ensure purity standards are maintained, reflecting the criticality of robust supply management in this early stage.
The midstream phase, where the API is actually synthesized via chelation, represents the core value addition. This stage requires significant technological investment in reactor systems, crystallization units, and stringent quality control testing to ensure the precise stoichiometry and high bioavailability characteristic of Bisglycinate. Efficiency here is paramount; optimizing reaction yields and reducing energy consumption directly translates to competitive pricing. High-purity APIs require additional purification steps, often involving advanced chromatography or recrystallization techniques, adding further value and justifying the premium pricing demanded by pharmaceutical and high-end nutraceutical buyers.
The downstream distribution channels are bifurcated into direct sales to large nutraceutical and pharmaceutical formulators and indirect sales through specialized chemical distributors. Direct sales are preferred for high-volume, customized orders, fostering stronger partnerships and deeper integration into the buyer's supply chain. Indirect channels offer broader market reach, particularly to smaller and medium-sized supplement companies globally. End-users then integrate the API into final product forms—capsules, powders, or functional beverages. The integrity of the distribution network, particularly regarding temperature and humidity control, is essential to maintain the API's stability until it reaches the final formulation plant.
Magnesium Bisglycinate API Market Potential Customers
The primary customers and buyers of Magnesium Bisglycinate API are concentrated in the health and wellness sectors, seeking high-quality, scientifically validated mineral ingredients. The largest segment comprises Nutraceutical Manufacturers, ranging from global leaders in dietary supplements to specialized brands focusing on sports nutrition, stress management, and sleep aids. These companies purchase the API in bulk for encapsulation, tableting, or blending into powdered mixes. Their purchasing decisions are driven by factors such as API purity, regulatory compliance (e.g., non-GMO, allergen-free certifications), competitive pricing, and the supplier's ability to provide extensive technical documentation and stability data to support their product claims.
A second major customer group includes Pharmaceutical Companies, both generics and branded firms, which utilize the API for specific therapeutic applications where highly absorbed magnesium is beneficial, such as in the management of certain neurological or cardiovascular conditions. This segment demands the highest quality—often USP or EP grade—with strict adherence to Good Manufacturing Practices (GMP). Procurement in this segment is characterized by long qualification periods, rigorous auditing, and requirement for detailed documentation regarding the API’s synthesis route, impurity profile, and batch-to-batch consistency, reflecting the strict regulatory environment of the pharmaceutical industry.
Furthermore, specialized segments such as Functional Food and Beverage Producers and Contract Development and Manufacturing Organizations (CDMOs) represent significant potential customers. Functional food producers integrate the API into items like fortified yogurts or health bars to enhance mineral content, focusing on solubility and taste neutrality. CDMOs, acting as intermediaries, purchase the API on behalf of multiple smaller brand owners, requiring robust technical support and flexible supply volumes. The expanding trend of personalized nutrition clinics and compounding pharmacies also contributes to the customer base, demanding smaller, highly traceable batches of the API.
| Report Attributes | Report Details |
|---|---|
| Market Size in 2026 | USD 450 Million |
| Market Forecast in 2033 | USD 1.05 Billion |
| Growth Rate | 12.5% CAGR |
| Historical Year | 2019 to 2024 |
| Base Year | 2025 |
| Forecast Year | 2026 - 2033 |
| DRO & Impact Forces |
|
| Segments Covered |
|
| Key Companies Covered | Albion Minerals, Balchem Corporation, Dr. Paul Lohmann GmbH KG, Merck KGaA, VITAL-CHEM Industry Co., Ltd., Parchem fine & specialty chemicals, Lianyungang Tongyuan Chemical Co., Ltd., Innophos, Tri-K Industries, Inc., Biocad, Jiaxing Keyuan Chemical Co., Ltd., Shaanxi Jinrui Natural Ingredients Co., Ltd., Hefei TNJ Chemical Industry Co., Ltd., Hebei Cangzhou Mighty Chemical Co., Ltd., Hubei Zhaoke Biochemical Co., Ltd. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Enquiry Before Buy | Have specific requirements? Send us your enquiry before purchase to get customized research options. Request For Enquiry Before Buy |
Magnesium Bisglycinate API Market Key Technology Landscape
The technology landscape for Magnesium Bisglycinate API manufacturing is defined by continuous advancements aimed at improving chelation efficiency, scalability, and purity, particularly focusing on reducing heavy metal contamination. The dominant technology remains traditional chemical synthesis, which involves reacting magnesium oxide or chloride with glycine under controlled temperature and pH conditions to facilitate the chelation process. Critical technological refinements in this established method include advanced reactor design for homogeneous mixing and precise control over crystallization parameters, which directly impacts the particle morphology and flowability of the final API powder. Optimization of filtration and drying techniques is also vital to remove residual solvents and ensure the API meets stringent stability requirements for long shelf life.
A significant emerging technological trend is the adoption of "Green Chemistry" principles, particularly leveraging enzymatic or bio-catalytic synthesis routes. These novel methods utilize highly specific enzymes to catalyze the chelation reaction, leading to fewer unwanted byproducts, reduced waste streams, and the ability to operate under milder conditions (lower temperature and pressure). While currently more expensive to implement than conventional methods, enzymatic synthesis offers substantial environmental benefits and can potentially yield a purer, more consistent product. Companies investing heavily in biotechnology are positioning themselves to capitalize on the future demand for sustainably produced APIs, especially as regulatory pressure regarding manufacturing environmental footprint increases globally.
Furthermore, technology related to analytical testing and quality assurance is rapidly evolving. High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) are standard for purity testing, but modern facilities are integrating automation and inline spectroscopic techniques (like Near-Infrared Spectroscopy) for continuous, real-time monitoring of the chelation process. This allows for immediate adjustments, ensuring every batch meets the precise specifications required by pharmacopeias. The implementation of advanced particle size reduction technologies, such as micronization, is also key, as smaller particle sizes can further enhance solubility and integration into complex formulation matrices, improving the overall consumer experience and product efficacy.
Regional Highlights
The global Magnesium Bisglycinate API market displays significant regional variation in growth drivers, regulatory stringency, and consumption patterns. Understanding these geographic nuances is critical for effective market strategy formulation and supply chain management.
- North America: This region maintains the largest market share, driven by a mature dietary supplement industry, high consumer awareness regarding micronutrient deficiencies, and strong disposable incomes allowing for premium product purchases. The U.S. market, in particular, is characterized by rigorous standards set by organizations like the FDA, pushing API manufacturers to maintain high purity and transparent sourcing. Innovation in sports nutrition and mental wellness supplements is concentrated here, ensuring continuous demand.
- Europe: Europe is a substantial consumer market, characterized by strict regulatory frameworks (EFSA regulations) concerning health claims and purity standards. Demand is driven by aging populations and a cultural emphasis on preventative medicine. Germany, the UK, and France are major consumption hubs, with a growing focus on organic and naturally sourced ingredients, favoring suppliers who can demonstrate sustainable production practices for the API.
- Asia Pacific (APAC): APAC is projected to exhibit the highest Compound Annual Growth Rate (CAGR) due to rapid urbanization, increasing health expenditure, and the proliferation of local pharmaceutical and nutraceutical manufacturing bases in China, India, and Southeast Asia. While price sensitivity remains a factor, the increasing middle class is rapidly shifting toward high-quality, imported supplement concepts, creating massive expansion opportunities, particularly for standard-grade APIs used in high-volume, affordable supplements.
- Latin America (LATAM): The LATAM market, while smaller, is growing steadily, propelled by improving economic conditions and increased accessibility to global supplement brands. Brazil and Mexico are key markets, showing robust demand for mineral supplements focused on general wellness and sports recovery. Market growth is heavily dependent on overcoming import barriers and navigating fragmented local regulatory landscapes.
- Middle East and Africa (MEA): This region is an emerging market, primarily driven by Gulf Cooperation Council (GCC) countries investing in healthcare infrastructure and rising awareness of nutritional deficiencies. Growth here is sensitive to global pricing fluctuations and requires careful logistics management due to varied climate conditions and import logistics challenges.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Magnesium Bisglycinate API Market.- Albion Minerals (A Balchem Company)
- Dr. Paul Lohmann GmbH KG
- Merck KGaA
- VITAL-CHEM Industry Co., Ltd.
- Parchem fine & specialty chemicals
- Lianyungang Tongyuan Chemical Co., Ltd.
- Innophos
- Tri-K Industries, Inc.
- Balchem Corporation
- Kyowa Hakko Bio Co., Ltd.
- Jiaxing Keyuan Chemical Co., Ltd.
- Shaanxi Jinrui Natural Ingredients Co., Ltd.
- Hefei TNJ Chemical Industry Co., Ltd.
- Hebei Cangzhou Mighty Chemical Co., Ltd.
- Hubei Zhaoke Biochemical Co., Ltd.
- Chemie Uetikon GmbH
- Jiangsu Baiao Chemical Co., Ltd.
- Yichun Wanshen Pharmaceutical Co., Ltd.
- NutriScience Innovations, LLC
- Norkem Group
Frequently Asked Questions
Analyze common user questions about the Magnesium Bisglycinate API market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the primary factor driving the demand for Magnesium Bisglycinate API?
The primary driver is the superior bioavailability of Magnesium Bisglycinate compared to common magnesium salts, leading to enhanced efficacy and significantly reduced gastrointestinal side effects. This makes it the preferred API for high-end nutraceutical formulations targeting optimal absorption for muscle, sleep, and nervous system support.
How does the purity grade of Magnesium Bisglycinate API influence its market value?
Purity grade directly correlates with market value; High Purity/Pharmaceutical Grade API commands a premium due to strict regulatory requirements (like USP/EP standards), extremely low heavy metal contamination, and necessity for detailed batch traceability, essential for pharmaceutical and clinical applications.
What major technological advancement is expected to impact the API synthesis in the near future?
The adoption of enzymatic or bio-catalytic synthesis methods is the major technological shift. This green chemistry approach offers potential advantages in achieving higher product specificity, reducing environmental waste, and ensuring compliance with stringent sustainability mandates, offering a competitive edge to innovators.
Which geographical region is anticipated to show the fastest growth rate for the market?
The Asia Pacific (APAC) region is projected to register the fastest CAGR, driven by rapid expansion of domestic supplement manufacturing capabilities, increasing consumer health awareness, and growing disposable incomes, particularly in populous countries such as China and India.
What are the main applications of Magnesium Bisglycinate API outside of general dietary supplements?
Key non-supplement applications include its use in specialized pharmaceutical preparations for neurological and cardiovascular support, integration into high-performance sports nutrition products for enhanced recovery, and fortification of functional foods designed to combat specific micronutrient deficiencies in target populations.
To check our Table of Contents, please mail us at: sales@marketresearchupdate.com
Research Methodology
The Market Research Update offers technology-driven solutions and its full integration in the research process to be skilled at every step. We use diverse assets to produce the best results for our clients. The success of a research project is completely reliant on the research process adopted by the company. Market Research Update assists its clients to recognize opportunities by examining the global market and offering economic insights. We are proud of our extensive coverage that encompasses the understanding of numerous major industry domains.
Market Research Update provide consistency in our research report, also we provide on the part of the analysis of forecast across a gamut of coverage geographies and coverage. The research teams carry out primary and secondary research to implement and design the data collection procedure. The research team then analyzes data about the latest trends and major issues in reference to each industry and country. This helps to determine the anticipated market-related procedures in the future. The company offers technology-driven solutions and its full incorporation in the research method to be skilled at each step.
The Company's Research Process Has the Following Advantages:
- Information Procurement
The step comprises the procurement of market-related information or data via different methodologies & sources.
- Information Investigation
This step comprises the mapping and investigation of all the information procured from the earlier step. It also includes the analysis of data differences observed across numerous data sources.
- Highly Authentic Source
We offer highly authentic information from numerous sources. To fulfills the client’s requirement.
- Market Formulation
This step entails the placement of data points at suitable market spaces in an effort to assume possible conclusions. Analyst viewpoint and subject matter specialist based examining the form of market sizing also plays an essential role in this step.
- Validation & Publishing of Information
Validation is a significant step in the procedure. Validation via an intricately designed procedure assists us to conclude data-points to be used for final calculations.
×
Request Free Sample:
Related Reports
Select License

Why Choose Us

We're cost-effective and Offered Best services:
We are flexible and responsive startup research firm. We adapt as your research requires change, with cost-effectiveness and highly researched report that larger companies can't match.

Information Safety
Market Research Update ensure that we deliver best reports. We care about the confidential and personal information quality, safety, of reports. We use Authorize secure payment process.

We Are Committed to Quality and Deadlines
We offer quality of reports within deadlines. We've worked hard to find the best ways to offer our customers results-oriented and process driven consulting services.

Our Remarkable Track Record
We concentrate on developing lasting and strong client relationship. At present, we hold numerous preferred relationships with industry leading firms that have relied on us constantly for their research requirements.

Best Service Assured
Buy reports from our executives that best suits your need and helps you stay ahead of the competition.

Customized Research Reports
Our research services are custom-made especially to you and your firm in order to discover practical growth recommendations and strategies. We don't stick to a one size fits all strategy. We appreciate that your business has particular research necessities.

Service Assurance
At Market Research Update, we are dedicated to offer the best probable recommendations and service to all our clients. You will be able to speak to experienced analyst who will be aware of your research requirements precisely.
Contact With Our Sales Team
Customer Testimonials
The content of the report is always up to the mark. Good to see speakers from expertise authorities.
Privacy requested , Managing Director
A lot of unique and interesting topics which are described in good manner.
Privacy requested, President
Well researched, expertise analysts, well organized, concrete and current topics delivered in time.
Privacy requested, Development Manager
